
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as halides of carbon without carbon-hydrogen and carbon-carbon bonds, and certain compounds of carbon with nitrogen and oxygen.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Organic farming, also known as ecological farming or biological farming, is an agricultural system that uses fertilizers of organic origin such as compost manure, green manure, and bone meal and places emphasis on techniques such as crop rotation and companion planting. It originated early in the 20th century in reaction to rapidly changing farming practices. Certified organic agriculture accounts for 70 million hectares globally, with over half of that total in Australia. Biological pest control, mixed cropping, and the fostering of insect predators are encouraged. Organic standards are designed to allow the use of naturally-occurring substances while prohibiting or strictly limiting synthetic substances. For instance, naturally-occurring pesticides such as pyrethrin are permitted, while synthetic fertilizers and pesticides are generally prohibited. Synthetic substances that are allowed include, for example, copper sulfate, elemental sulfur, and veterinary drugs. Genetically modified organisms, nanomaterials, human sewage sludge, plant growth regulators, hormones, and antibiotic use in livestock husbandry are prohibited. Organic farming advocates claim advantages in sustainability, openness, self-sufficiency, autonomy and independence, health, food security, and food safety.

In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles with others to form a protein complex. Large assemblies of proteins such as viruses often use a small number of types of protein subunits as building blocks.

Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.
The Aldol–Tishchenko reaction is a tandem reaction involving an aldol reaction and a Tishchenko reaction. In organic synthesis it is a method to convert aldehydes and ketones into 1,3-hydroxyl compounds. The reaction sequence in many examples starts from conversion of a ketone into an enolate by action of lithium diisopropylamide (LDA), to which a suitable aldehyde is added. The resulting mono-ester diol is then converted into the diol by a hydrolysis step. With both the acetyl trimethylsilane and propiophenone as reactants, the diol is obtained as a pure diastereoisomer.

Thiocyanic acid is a chemical compound with the formula HSCN and structure H−S−C≡N, which exists as a tautomer with isothiocyanic acid. The isothiocyanic acid tautomer tends to dominate with the compound being about 95% isothiocyanic acid in the vapor phase.

Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many natural products.

2-Ethylhexyl salicylate also known as octisalate or octyl salicylate, is an organic compound used as an ingredient in sunscreens and cosmetics to absorb UVB (ultraviolet) rays from the sun. It is an ester formed by the condensation of salicylic acid with 2-ethylhexanol. It is a colorless oily liquid with a slight floral odor.
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature. It has the odor of ozone. Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.

Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs.
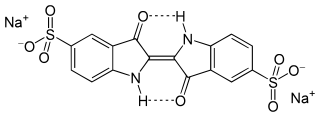
Indigo carmine, or 5,5′-indigodisulfonic acid sodium salt, is an organic salt derived from indigo by aromatic sulfonation, which renders the compound soluble in water. It is approved for use as a food colorant in the United States and European Union to produce a blue color. It has the E number E132, and is named Blue No. 2 by the Federal Food, Drug, and Cosmetic Act. It is also a pH indicator.

Jewish Babylonian Aramaic was the form of Middle Aramaic employed by writers in Lower Mesopotamia between the fourth and eleventh centuries. It is most commonly identified with the language of the Babylonian Talmud, the Targum Onqelos, and of post-Talmudic (Gaonic) literature, which are the most important cultural products of Babylonian Jews. The most important epigraphic sources for the dialect are the hundreds of inscriptions on incantation bowls.

No. 466 Squadron RAAF was a Royal Australian Air Force (RAAF) bomber squadron during World War II. Formed in the United Kingdom in late 1942, the squadron undertook combat operations in Europe until the end of the war, flying heavy bomber aircraft. Following the conclusion of hostilities with Germany, the squadron began retraining to undertake operations in the Pacific against the Japanese, but the war came to an end before it left the UK. In late 1945, the squadron was disbanded.

A bottom feeder is an aquatic animal that feeds on or near the bottom of a body of water. Biologists often use the terms benthos—particularly for invertebrates such as shellfish, crabs, crayfish, sea anemones, starfish, snails, bristleworms and sea cucumbers—and benthivore or benthivorous, for fish and invertebrates that feed on material from the bottom. However the term benthos includes all aquatic life that lives on or near the bottom, which means it also includes non-animals, such as plants and algae. Biologists also use specific terms that refer to bottom feeding fish, such as demersal fish, groundfish, benthic fish and benthopelagic fish. Examples of bottom feeding fish species groups are flatfish, eels, cod, haddock, bass, grouper, carp, bream (snapper) and some species of catfish, sharks.
National Route 466 is a national highway in Japan connecting Setagaya and Hodogaya-ku, Yokohama, with a total length of 18.4 kilometers (11.4 mi).
German submarine U-466 was a Type VIIC U-boat built for Nazi Germany's Kriegsmarine for service during World War II. She was scuttled at sea on 19 August 1944.

USS LST-466 was a United States Navy LST-1-class tank landing ship used in the Asiatic-Pacific Theater during World War II. As with many of her class, the ship was never named. Instead, she was referred to by her hull designation.

NGC 466 is a lenticular galaxy located about 227 million light-years away from Earth in the constellation Tucana. NGC 466 was discovered by astronomer John Herschel on October 3, 1836.

















