
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Fire is the rapid oxidation of a material in the exothermic chemical process of combustion, releasing heat, light, and various reaction products. At a certain point in the combustion reaction, called the ignition point, flames are produced. The flame is the visible portion of the fire. Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen. If hot enough, the gases may become ionized to produce plasma. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's intensity will be different.

A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal micro calorimeters, titration calorimeters and accelerated rate calorimeters are among the most common types. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. It is one of the measurement devices used in the study of thermodynamics, chemistry, and biochemistry.

Thermite is a pyrotechnic composition of metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties are not explosive, but can create brief bursts of heat and high temperature in a small area. Its form of action is similar to that of other fuel-oxidizer mixtures, such as black powder.

A kerosene lamp is a type of lighting device that uses kerosene as a fuel. Kerosene lamps have a wick or mantle as light source, protected by a glass chimney or globe; lamps may be used on a table, or hand-held lanterns may be used for portable lighting. Like oil lamps, they are useful for lighting without electricity, such as in regions without rural electrification, in electrified areas during power outages, at campsites, and on boats. There are three types of kerosene lamp: flat-wick, central-draft, and mantle lamp. Kerosene lanterns meant for portable use have a flat wick and are made in dead-flame, hot-blast, and cold-blast variants.

A flame is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density, they are then considered plasma.

A catalytic converter is an exhaust emission control device which converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually used with internal combustion engines fueled by gasoline or diesel, including lean-burn engines, and sometimes on kerosene heaters and stoves.
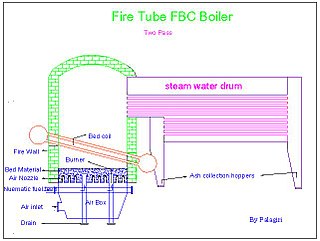
Fluidized bed combustion (FBC) is a combustion technology used to burn solid fuels.

Thorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in colour. Also known as thoria, it is mainly a by-product of lanthanide and uranium production. Thorianite is the name of the mineralogical form of thorium dioxide. It is moderately rare and crystallizes in an isometric system. The melting point of thorium oxide is 3300 °C – the highest of all known oxides. Only a few elements (including tungsten and carbon) and a few compounds (including tantalum carbide) have higher melting points. All thorium compounds, including the dioxide, are radioactive because there are no stable isotopes of thorium.

Basic oxygen steelmaking, also known as Linz-Donawitz steelmaking or the oxygen converter process, is a method of primary steelmaking in which carbon-rich molten pig iron is made into steel. Blowing oxygen through molten pig iron lowers the carbon content of the alloy and changes it into low-carbon steel. The process is known as basic because fluxes of burnt lime or dolomite, which are chemical bases, are added to promote the removal of impurities and protect the lining of the converter.
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition.

Hand warmers are small, often disposable, packets that produce heat to warm cold hands. They are used throughout the world in a variety of ways, including outdoor recreation, manual labor, and homelessness.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.

A flare or decoy flare is an aerial infrared countermeasure used by an aircraft to counter an infrared homing ("heat-seeking") surface-to-air missile or air-to-air missile. Flares are commonly composed of a pyrotechnic composition based on magnesium or another hot-burning metal, with burning temperature equal to or hotter than engine exhaust. The aim is to make the infrared-guided missile seek out the heat signature from the flare rather than the aircraft's engines.

Oxy-fuel welding and oxy-fuel cutting are processes that use fuel gases and oxygen to weld or cut metals. French engineers Edmond Fouché and Charles Picard became the first to develop oxygen-acetylene welding in 1903. Pure oxygen, instead of air, is used to increase the flame temperature to allow localized melting of the workpiece material in a room environment. A common propane/air flame burns at about 2,250 K, a propane/oxygen flame burns at about 2,526 K, an oxyhydrogen flame burns at 3,073 K and an acetylene/oxygen flame burns at about 3,773 K.
Delay composition, also called delay charge or delay train, is a pyrotechnic composition, a sort of pyrotechnic initiator, a mixture of oxidizer and fuel that burns in a slow, constant rate that should not be significantly dependent on temperature and pressure. Delay compositions are used to introduce a delay into the firing train, e.g. to properly sequence firing of fireworks, to delay firing of ejection charges in e.g. model rockets, or to introduce a few seconds of time between triggering a hand grenade and its explosion. Typical delay times range between several milliseconds and several seconds.
A pyrotechnic heat source, also called heat pellet, is a pyrotechnic device based on a pyrotechnic composition with a suitable igniter. Its role is to produce controlled amount of heat. Pyrotechnic heat sources are usually based on thermite-like fuel-oxidizer compositions with slow burn rate, high production of heat at desired temperature, and low to zero production of gases.

Charcoal is a lightweight black carbon residue produced by strongly heating wood in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is supplied by burning part of the starting material itself, with a limited supply of oxygen. The material can also be heated in a closed retort. Modern "charcoal" briquettes used for outdoor cooking may contain many other additives, e.g. coal.

A spark is an incandescent particle. Sparks may be produced by pyrotechnics, by metalworking or as a by-product of fires, especially when burning wood.

An internal combustion engine is a heat engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons, turbine blades, a rotor, or a nozzle. This force moves the component over a distance, transforming chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to.

















