Histocompatibility, or tissue compatibility, is the property of having the same, or sufficiently similar, alleles of a set of genes called human leukocyte antigens (HLA), or major histocompatibility complex (MHC). Each individual expresses many unique HLA proteins on the surface of their cells, which signal to the immune system whether a cell is part of the self or an invading organism. T cells recognize foreign HLA molecules and trigger an immune response to destroy the foreign cells. Histocompatibility testing is most relevant for topics related to whole organ, tissue, or stem cell transplants, where the similarity or difference between the donor's HLA alleles and the recipient's triggers the immune system to reject the transplant. The wide variety of potential HLA alleles lead to unique combinations in individuals and make matching difficult.

The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are called MHC molecules.

The human leukocyte antigen (HLA) system or complex is a complex of genes on chromosome 6 in humans which encode cell-surface proteins responsible for regulation of the immune system. The HLA system is also known as the human version of the major histocompatibility complex (MHC) found in many animals.

Transplant rejection occurs when transplanted tissue is rejected by the recipient's immune system, which destroys the transplanted tissue. Transplant rejection can be lessened by determining the molecular similitude between donor and recipient and by use of immunosuppressant drugs after transplant.
Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood in order to replicate inside of a patient and to produce additional normal blood cells. It may be autologous, allogeneic or syngeneic.

Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.

Xenotransplantation, or heterologous transplant, is the transplantation of living cells, tissues or organs from one species to another. Such cells, tissues or organs are called xenografts or xenotransplants. It is contrasted with allotransplantation, syngeneic transplantation or isotransplantation and autotransplantation. Xenotransplantation is an artificial method of creating an animal-human chimera, that is, a human with a subset of animal cells. In contrast, an individual where each cell contains genetic material from a human and an animal is called a human–animal hybrid.

A serotype or serovar is a distinct variation within a species of bacteria or virus or among immune cells of different individuals. These microorganisms, viruses, or cells are classified together based on their surface antigens, allowing the epidemiologic classification of organisms to the subspecies level. A group of serovars with common antigens is called a serogroup or sometimes serocomplex.

HLA-DQ (DQ) is a cell surface receptor protein found on antigen-presenting cells. It is an αβ heterodimer of type MHC class II. The α and β chains are encoded by two loci, HLA-DQA1 and HLA-DQB1, that are adjacent to each other on chromosome band 6p21.3. Both α-chain and β-chain vary greatly. A person often produces two α-chain and two β-chain variants and thus 4 isoforms of DQ. The DQ loci are in close genetic linkage to HLA-DR, and less closely linked to HLA-DP, HLA-A, HLA-B and HLA-C.

Minor histocompatibility antigen are peptides presented on the cellular surface of donated organs that are known to give an immunological response in some organ transplants. They cause problems of rejection less frequently than those of the major histocompatibility complex (MHC). Minor histocompatibility antigens (MiHAs) are diverse, short segments of proteins and are referred to as peptides. These peptides are normally around 9-12 amino acids in length and are bound to both the major histocompatibility complex (MHC) class I and class II proteins. Peptide sequences can differ among individuals and these differences arise from SNPs in the coding region of genes, gene deletions, frameshift mutations, or insertions. About a third of the characterized MiHAs come from the Y chromosome. Prior to becoming a short peptide sequence, the proteins expressed by these polymorphic or diverse genes need to be digested in the proteasome into shorter peptides. These endogenous or self peptides are then transported into the endoplasmic reticulum with a peptide transporter pump called TAP where they encounter and bind to the MHC class I molecule. This contrasts with MHC class II molecules's antigens which are peptides derived from phagocytosis/endocytosis and molecular degradation of non-self entities' proteins, usually by antigen-presenting cells. MiHA antigens are either ubiquitously expressed in most tissue like skin and intestines or restrictively expressed in the immune cells.

HLA-DR52 is an HLA-DR serotype that recognizes gene products of HLA-DRB3 locus. Three allele groups can produce 35 isoforms.

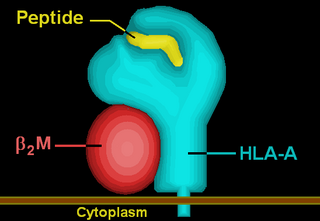
HLA-A*02 (A*02) is a human leukocyte antigen serotype within the HLA-A serotype group. The serotype is determined by the antibody recognition of the α2 domain of the HLA-A α-chain. For A*02, the α chain is encoded by the HLA-A*02 gene and the β chain is encoded by the B2M locus. In 2010 the World Health Organization Naming Committee for Factors of the HLA System revised the nomenclature for HLAs. Before this revision, HLA-A*02 was also referred to as HLA-A2, HLA-A02, and HLA-A*2.
HLA-A69 (A69) is a human leukocyte antigen serotype within HLA-A serotype group. The serotype is determined by the antibody recognition of α69 subset of HLA-A α-chains. For A69, the alpha "A" chain are encoded by the HLA-A*69 allele group and the β-chain are encoded by B2M locus. This group currently is dominated by A*6901. A69 and A*69 are almost synonymous in meaning. A69 is a split antigen of the broad antigen serotype A28. A69 is a sister serotype of A68.

HLA-A33 (A33) is a human leukocyte antigen serotype within HLA-A serotype group. The serotype is determined by the antibody recognition of α33 subset of HLA-A α-chains. For A33, the alpha "A" chain are encoded by the HLA-A*33 allele group and the β-chain are encoded by B2M locus. A33 and A*33 are almost synonymous in meaning. A33 is a split antigen of the broad antigen serotype A19. A33 is a sister serotype of A29, A30, A31, A32, and A74.
A panel-reactive antibody (PRA) is a group of antibodies in a test serum that are reactive against any of several known specific antigens in a panel of test leukocytes or purified HLA antigens from cells. It is an immunologic metric routinely performed by clinical laboratories on the blood of people awaiting organ transplantation.

Paul Ichiro Terasaki was an American scientist in the field of human organ transplant technology, and professor emeritus of surgery at UCLA School of Medicine.
Human leukocyte antigens (HLA) began as a list of antigens identified as a result of transplant rejection. The antigens were initially identified by categorizing and performing massive statistical analyses on interactions between blood types. This process is based upon the principle of serotypes. HLA are not typical antigens, like those found on surface of infectious agents. HLAs are alloantigens, they vary from individual to individual as a result of genetic differences. An organ called the thymus is responsible for ensuring that any T-cells that attack self proteins are not allowed to live. In essence, every individual's immune system is tuned to the specific set of HLA and self proteins produced by that individual; where this goes awry is when tissues are transferred to another person. Since individuals almost always have different "banks" of HLAs, the immune system of the recipient recognizes the transplanted tissue as non-self and destroys the foreign tissue, leading to transplant rejection. It was through the realization of this that HLAs were discovered.
HLA A1-B8 is a multigene haplotype that covers the MHC Class I region of the human major histocompatibility complex on chromosome 6. A multigene haplotype is set of inherited alleles covering several genes, or gene-alleles; common multigene haplotypes are generally the result of identity by descent from a common ancestor. Chromosomal recombination fragments multigene haplotypes as the distance to that ancestor increases in number of generations.
HLA B7-DR15-DQ6 is a multigene haplotype that covers a majority of the human major histocompatibility complex on chromosome 6. A multigene haplotype is set of inherited alleles covering several genes, or gene-alleles, common multigene haplotypes are generally the result of descent by common ancestry. Chromosomal recombination fragments multigene haplotypes as the distance to that ancestor increases in number of generations.
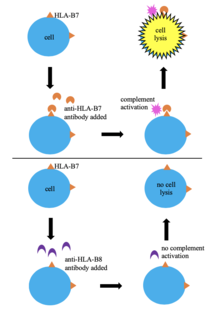
Complement-dependent cytotoxicity (CDC) is an effector function of IgG and IgM antibodies. When they are bound to surface antigen on target cell, the classical complement pathway is triggered by bonding protein C1q to these antibodies, resulting in formation of a membrane attack complex (MAC) and target cell lysis.