A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as "tickle" or "tickle 4", as a phonetic representation of the symbols of its molecular formula.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

1,4,7-Triazacyclononane, known as "TACN" which is pronounced "tack-en," is an aza-crown ether with the formula (C2H4NH)3. TACN is derived, formally speaking, from cyclononane by replacing three equidistant CH2 groups with NH groups. TACN is one of the oligomers derived from aziridine, C2H4NH. Other members of the series include piperazine, C4H8(NH)2, and the cyclic tetramer 1,4,7,10-tetraazacyclododecane.

Titanium(II) chloride is the chemical compound with the formula TiCl2. The black solid has been studied only moderately, probably because of its high reactivity. Ti(II) is a strong reducing agent: it has a high affinity for oxygen and reacts irreversibly with water to produce H2. The usual preparation is the thermal disproportionation of TiCl3 at 500 °C. The reaction is driven by the loss of volatile TiCl4:
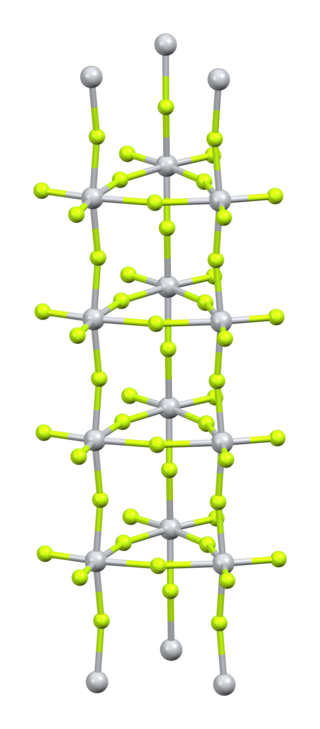
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

The vanadyl or oxovanadium(IV) cation, VO2+, is a functional group that is common in the coordination chemistry of vanadium. Complexes containing this functional group are characteristically blue and paramagnetic. A triple bond is proposed to exist between the V4+ and O2− centers. The description of the bonding in the vanadyl ion was central to the development of modern ligand-field theory.
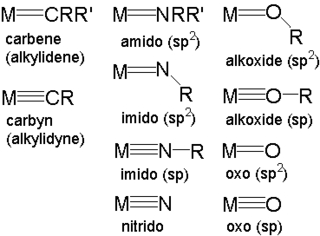
In organometallic chemistry, a metal–ligand multiple bond describes the interaction of certain ligands with a metal with a bond order greater than one. Coordination complexes featuring multiply bonded ligands are of both scholarly and practical interest. transition metal carbene complexes catalyze the olefin metathesis reaction. Metal oxo intermediates are pervasive in oxidation catalysis.
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early transition metals such as titanium, niobium, and tungsten. Oxophilicity is often stated to be related to the hardness of the element, within the HSAB theory, but it has been shown that oxophilicity depends more on the electronegativity and effective nuclear charge of the element than on its hardness. This explains why the early transition metals, whose electronegativities and effective nuclear charges are low, are very oxophilic. Many main group compounds are also oxophilic, such as derivatives of aluminium, silicon, and phosphorus(III). The handling of oxophilic compounds often requires air-free techniques.
Dioxygen complexes are coordination compounds that contain O2 as a ligand. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin, hemoglobin, hemerythrin, and hemocyanin. Several transition metals form complexes with O2, and many of these complexes form reversibly. The binding of O2 is the first step in many important phenomena, such as cellular respiration, corrosion, and industrial chemistry. The first synthetic oxygen complex was demonstrated in 1938 with cobalt(II) complex reversibly bound O2.
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes.
A transition metal oxo complex is a coordination complex containing an oxo ligand. Formally O2-, an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) as bridging ligands (Fig. 1). Oxo ligands stabilize high oxidation states of a metal. They are also found in several metalloproteins, for example in molybdenum cofactors and in many iron-containing enzymes. One of the earliest synthetic compounds to incorporate an oxo ligand is potassium ferrate (K2FeO4), which was likely prepared by Georg E. Stahl in 1702.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).
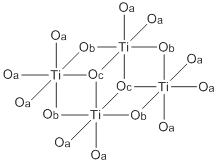
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a commercially available colorless liquid that is soluble in organic solvents but hydrolyzes readily. Its structure is more complex than suggested by its empirical formula. Like other alkoxides of titanium(IV) and zirconium(IV), it finds used in organic synthesis and materials science.

Titanium perchlorate is a molecular compound of titanium and perchlorate groups with formula Ti(ClO4)4. Anhydrous titanium perchlorate decomposes explosively at 130 °C and melts at 85 °C with a slight decomposition. It can sublime in a vacuum as low as 70 °C, and can form vapour at up to 120°. Titanium perchlorate is quite volatile. It has density 2.35. It decomposes to TiO2, ClO2 and dioxygen O2 Also TiO(ClO4)2 is formed during decomposition.
Christopher "Kit" Colin Cummins is an American chemist, currently the Henry Dreyfus Professor at the Massachusetts Institute of Technology. He has made contributions to the coordination chemistry of transition metal nitrides, phosphides, and carbides.

Titanyl sulfate is the inorganic compound with the formula TiOSO4. It is a white solid that forms by treatment of titanium dioxide with fuming sulfuric acid. It hydrolyzes to a gel of hydrated titanium dioxide. The structure consists of dense polymeric network with tetrahedral sulfur and octahedral titanium centers. The six ligands attached to titanium are derived from four different sulfate moieties and a bridging oxide. A monohydrate is also known, being prepared similarly to the anhydrous material. In the hydrate, one Ti–OS bond is replaced by Ti–OH2.
Karl Wieghardt is a German inorganic chemist and emeritus director of the Max Planck Institute for Chemical Energy Conversion in Mülheim. He was active in the preparation and detailed characterization of models for iron and manganese metalloenzymes, metal complexes of noninnocent ligands, and magnetic interactions in polynuclear metal complexes.
The +4 oxidation state dominates titanium chemistry, but compounds in the +3 oxidation state are also numerous. Commonly, titanium adopts an octahedral coordination geometry in its complexes, but tetrahedral TiCl4 is a notable exception. Because of its high oxidation state, titanium(IV) compounds exhibit a high degree of covalent bonding.











