Galactose, sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule.

Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated.
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.
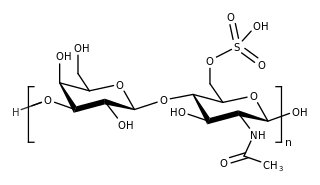
Keratan sulfate (KS), also called keratosulfate, is any of several sulfated glycosaminoglycans that have been found especially in the cornea, cartilage, and bone. It is also synthesized in the central nervous system where it participates both in development and in the glial scar formation following an injury. Keratan sulfates are large, highly hydrated molecules which in joints can act as a cushion to absorb mechanical shock.

Glycosyltransferases are enzymes that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based.

N-Acetylgalactosamine (GalNAc), is an amino sugar derivative of galactose.
The galactose permease or GalP found in Escherichia coli is an integral membrane protein involved in the transport of monosaccharides, primarily hexoses, for utilization by E. coli in glycolysis and other metabolic and catabolic pathways (3,4). It is a member of the Major Facilitator Super Family (MFS) and is homologue of the human GLUT1 transporter (4). Below you will find descriptions of the structure, specificity, effects on homeostasis, expression, and regulation of GalP along with examples of several of its homologues.

The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.

Peanut agglutinin (PNA) is plant lectin protein derived from the fruits of Arachis hypogaea. Peanut agglutinin may also be referred to as Arachis hypogaea lectin. Lectins recognise and bind particular sugar sequences in carbohydrates; peanut agglutinin binds the carbohydrate sequence Gal-β(1-3)-GalNAc. The name "peanut agglutinin" originates from its ability to stick together (agglutinate) cells, such as neuraminidase-treated erythrocytes, which have glycoproteins or glycolipids on their surface which include the Gal-β(1-3)-GalNAc carbohydrate sequence.

Alpha-N-acetylgalactosaminide alpha-2,6-sialyltransferase 1 is an enzyme that in humans is encoded by the ST6GALNAC1 gene. This enzyme adds a N-acetylneuraminic acid to an O-linked N-acetylgalactosamine (GalNAc) on a peptide/proteins with an α2-6 linkage to produce the sialyl-Tn antigen. It has been shown that the enzyme prefers threonine over serine containing GalNAc residues.
Histo-blood group ABO system transferase is an enzyme with glycosyltransferase activity, which is encoded by the ABO gene in humans. It is ubiquitously expressed in many tissues and cell types. ABO determines the ABO blood group of an individual by modifying the oligosaccharides on cell surface glycoproteins. Variations in the sequence of the protein between individuals determine the type of modification and the blood group. The ABO gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.
Arabinogalactan, also known as galactoarabinan, larch arabinogalactan, and larch gum, is a biopolymer consisting of arabinose and galactose monosaccharides. Two classes of arabinogalactans are found in nature: plant arabinogalactan and microbial arabinogalactan. In plants, it is a major component of many gums, including gum arabic and gum ghatti. It is often found attached to proteins, and the resulting arabinogalactan protein (AGP) functions as both an intercellular signaling molecule and a glue to seal plant wounds.
Core 1 synthase, glycoprotein-N-acetylgalactosamine 3-beta-galactosyltransferase, 1, also known as C1GALT1, is an enzyme which in humans is encoded by the C1GALT1 gene.

C-type lectin domain family 10 member A (CLEC10A) also designated as CD301 is a protein that in humans is encoded by the CLEC10A gene. CLEC10A is part of the C-type lectin superfamily and binds to N-Acetylgalactosamine (GalNAc). It is mainly expressed on myeloid cells and also on oocytes and very early stages of embryogenesis. CLEC10A is used as a marker of the CD1c+ dendritic cell subgroup, also called cDC2. The actions of CLEC10A are diverse, depending on the ligand and environment.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.

Galactose-α-1,3-galactose, commonly known as alpha gal and the Galili antigen, is a carbohydrate found in most mammalian cell membranes. It is not found in catarrhines, including humans, who have lost the GGTA1 gene. Their immune systems recognize it as a foreign body and produce xenoreactive immunoglobulin M (IgM) antibodies, leading to organ rejection after transplantation.
Alpha-gal syndrome (AGS), also known as alpha-gal allergy or mammalian meat allergy (MMA), is a type of acquired allergy characterized by a delayed onset of symptoms after ingesting mammalian meat. The condition results from past exposure to certain tick bites. It was first reported in 2002. Symptoms of the allergy vary greatly between individuals and include rash, hives, nausea or vomiting, difficulty breathing, drop in blood pressure, dizziness or faintness, diarrhea, severe stomach pain, and possible anaphylaxis.

Glycan-Protein interactions represent a class of biomolecular interactions that occur between free or protein-bound glycans and their cognate binding partners. Intramolecular glycan-protein (protein-glycan) interactions occur between glycans and proteins that they are covalently attached to. Together with protein-protein interactions, they form a mechanistic basis for many essential cell processes, especially for cell-cell interactions and host-cell interactions. For instance, SARS-CoV-2, the causative agent of COVID-19, employs its extensively glycosylated spike (S) protein to bind to the ACE2 receptor, allowing it to enter host cells. The spike protein is a trimeric structure, with each subunit containing 22 N-glycosylation sites, making it an attractive target for vaccine search.
The enterobacterial common antigen (ECA) is a carbohydrate antigen found in the outer membrane of many Enterobacterales species. The antigen is unanimously absent from other gram-negative and gram-positive bacteria. Aeromonas hydrophila 209A is the only organism outside of Enterobacterales that expresses the ECA. More studies are needed to explain the presence of the antigen in this species as no other strains of this species express the antigen. The ECA is a polysaccharide made of repeating units of trisaccharides. The functions of these units have very few proven functions. Some evidence indicates role in pathogenicity in the bacteria that present the ECA. There are three separate types of ECA these include ECAPG, ECALPS, and ECACYC, each have different lengths. The synthesis of the ECA is controlled by the wec operon and has a 12-step synthesis which is described below. Due to the lack of proven function of the ECA, any clinical significance is hard to define however, some evidence suggests that human serum has antibodies against ECA.











