Related Research Articles

Nutrition is the biochemical and physiological process by which an organism uses food to support its life. It provides organisms with nutrients, which can be metabolized to create energy and chemical structures. Failure to obtain sufficient nutrients causes malnutrition. Nutritional science is the study of nutrition, though it typically emphasizes human nutrition.
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excreted by cells to create non-cellular structures, such as hair, scales, feathers, or exoskeletons. Some nutrients can be metabolically converted to smaller molecules in the process of releasing energy, such as for carbohydrates, lipids, proteins, and fermentation products, leading to end-products of water and carbon dioxide. All organisms require water. Essential nutrients for animals are the energy sources, some of the amino acids that are combined to create proteins, a subset of fatty acids, vitamins and certain minerals. Plants require more diverse minerals absorbed through roots, plus carbon dioxide and oxygen absorbed through leaves. Fungi live on dead or living organic matter and meet nutrient needs from their host.

A period on the periodic table is a row of chemical elements. All elements in a row have the same number of electron shells. Each next element in a period has one more proton and is less metallic than its predecessor. Arranged this way, elements in the same group (column) have similar chemical and physical properties, reflecting the periodic law. For example, the halogens lie in the second-to-last group and share similar properties, such as high reactivity and the tendency to gain one electron to arrive at a noble-gas electronic configuration. As of 2022, a total of 118 elements have been discovered and confirmed.

In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life. However, the four major structural elements in the human body by weight (CHON), are usually not included in lists of major nutrient minerals. These four elements compose about 96% of the weight of the human body, and major minerals (macrominerals) and minor minerals compose the remainder.

Trace metals are the metals subset of trace elements; that is, metals normally present in small but measurable amounts in animal and plant cells and tissues and that are a necessary part of nutrition and physiology. Some biometals are trace metals. Ingestion of, or exposure to, excessive quantities can be toxic. However, insufficient plasma or tissue levels of certain trace metals can cause pathology, as is the case with iron.

Plant nutrition is the study of the chemical elements and compounds necessary for plant growth and reproduction, plant metabolism and their external supply. In its absence the plant is unable to complete a normal life cycle, or that the element is part of some essential plant constituent or metabolite. This is in accordance with Justus von Liebig’s law of the minimum. The total essential plant nutrients include seventeen different elements: carbon, oxygen and hydrogen which are absorbed from the air, whereas other nutrients including nitrogen are typically obtained from the soil.

Phytochemicals are chemical compounds produced by plants, generally to help them resist fungi, bacteria and plant virus infections, and also consumption by insects and other animals. The name comes from Greek φυτόν (phyton) 'plant'. Some phytochemicals have been used as poisons and others as traditional medicine.

Plant physiology is a subdiscipline of botany concerned with the functioning, or physiology, of plants. Closely related fields include plant morphology, plant ecology, phytochemistry, cell biology, genetics, biophysics and molecular biology.
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins.

Biometals are metals normally present, in small but important and measurable amounts, in biology, biochemistry, and medicine. The metals copper, zinc, iron, and manganese are examples of metals that are essential for the normal functioning of most plants and the bodies of most animals, such as the human body. A few are present in relatively larger amounts, whereas most others are trace metals, present in smaller but important amounts. Approximately 2/3 of the existing periodic table is composed of metals with varying properties, accounting for the diverse ways in which metals have been utilized in nature and medicine.
In biochemistry, an ultratrace element is a chemical element that normally comprises less than one microgram per gram of a given organism, but which plays a significant role in its metabolism.
Body composition may be analyzed in various ways. This can be done in terms of the chemical elements present, or by molecular type e.g., water, protein, fats, hydroxylapatite, carbohydrates and DNA. In terms of tissue type, the body may be analyzed into water, fat, connective tissue, muscle, bone, etc. In terms of cell type, the body contains hundreds of different types of cells, but notably, the largest number of cells contained in a human body are not human cells, but bacteria residing in the normal human gastrointestinal tract.

Manganese is an essential biological element in all organisms. It is used in many enzymes and proteins It is essential in plants.
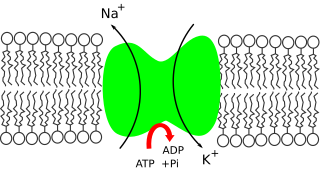
Sodium ions are necessary in small amounts for some types of plants, but sodium as a nutrient is more generally needed in larger amounts by animals, due to their use of it for generation of nerve impulses and for maintenance of electrolyte balance and fluid balance. In animals, sodium ions are necessary for the aforementioned functions and for heart activity and certain metabolic functions. The health effects of salt reflect what happens when the body has too much or too little sodium. Characteristic concentrations of sodium in model organisms are: 10 mM in E. coli, 30 mM in budding yeast, 10 mM in mammalian cell and 100 mM in blood plasma.
Chromium toxicity refers to any poisonous toxic effect in an organism or cell that results from exposure to specific forms of chromium—especially hexavalent chromium. Hexavalent chromium and its compounds are toxic when inhaled or ingested. Trivalent chromium is a trace mineral that is essential to human nutrition. There is a hypothetical risk of genotoxicity in humans if large amounts of trivalent chromium were somehow able to enter living cells, but normal metabolism and cell function prevent this.
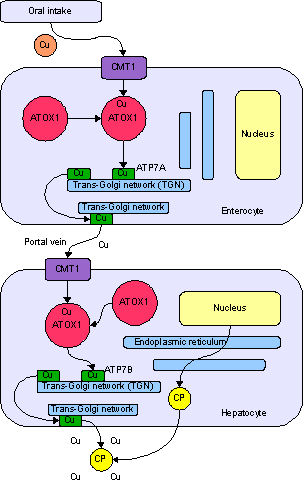
Copper is an essential trace element that is vital to the health of all living things. In humans, copper is essential to the proper functioning of organs and metabolic processes. The human body has complex homeostatic mechanisms which attempt to ensure a constant supply of available copper, while eliminating excess copper whenever this occurs. However, like all essential elements and nutrients, too much or too little nutritional ingestion of copper can result in a corresponding condition of copper excess or deficiency in the body, each of which has its own unique set of adverse health effects.

Although it is toxic in large doses, selenium is an essential micronutrient for animals. In plants, it sometimes occurs in toxic amounts as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase. Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−).
A large fraction of the chemical elements that occur naturally on the earth's surface are essential to the structure and metabolism of living things. Four of these elements are essential to every living thing and collectively make up 99% of the mass of protoplasm. Phosphorus and sulfur are also common essential elements, essential to the structure of nucleic acids and amino acids, respectively. Chlorine, potassium, magnesium, calcium and phosphorus have important roles due to their ready ionization and utility in regulating membrane activity and osmotic potential. The remaining elements found in living things are primarily metals that play a role in determining protein structure. Examples include iron, essential to hemoglobin; and magnesium, essential to chlorophyll. Some elements are essential only to certain taxonomic groups of organisms, particularly the prokaryotes. For instance, the lanthanide series rare earths are essential for methanogens. As shown in the following table, there is strong evidence that 19 of the elements are essential to all living things, and another 17 are essential to some taxonomic groups. Of these 17, most have not been extensively studied, and their biological importance may be greater than currently supposed.
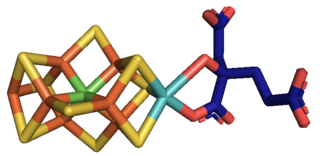
Molybdenum is an essential element in most organisms. It is most notably present in nitrogenase which is an essential part of nitrogen fixation.

Cobalt is essential to the metabolism of all animals. It is a key constituent of cobalamin, also known as vitamin B12, the primary biological reservoir of cobalt as an ultratrace element. Bacteria in the stomachs of ruminant animals convert cobalt salts into vitamin B12, a compound which can only be produced by bacteria or archaea. A minimal presence of cobalt in soils therefore markedly improves the health of grazing animals, and an uptake of 0.20 mg/kg a day is recommended because they have no other source of vitamin B12.
References
- ↑ Bhattacharya, Preeti Tomar; Misra, Satya Ranjan; Hussain, Mohsina (2016-06-28). "Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review". Scientifica. 2016: 1–12. doi: 10.1155/2016/5464373 . PMC 4940574 . PMID 27433374.
- ↑ "Definition of Trace element". www.merriam-webster.com. Retrieved 30 June 2023.
- ↑ "What are Trace Elements ?" (PDF).
- ↑ Bowen, Humphrey John Moule (1966). Trace elements in biochemistry. Academic Press. ISBN 9780121209506.
- 1 2 3 Soto-Jiménez, Martin (December 2011). "Trace element trophic transfer in aquatic food webs". Hidrobiológica. 21 (3): 239–248. ISSN 0188-8897 . Retrieved 5 November 2018.
- ↑ Shier, Butler, Lewis, David, Jackie, Ricki (2016). Hole's Human Anatomy Fourteenth Edition. New York: McGraw Hill Education. p. 59. ISBN 978-0-07-802429-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Rhodes CJ, Valko M (November 2022). "Essential metals in health and disease". Chem Biol Interact. 367: 110173. doi: 10.1016/j.cbi.2022.110173 . PMID 36152810.
- ↑ "Essential Elements for Life". saylordotorg.github.io. Retrieved 2023-02-13.