
A Schiff base (named after Hugo Schiff) is a compound with the general structure R1R2C=NR' (R' ≠ H). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimines depending on their structure. The term is often synonymous with azomethine which refers specifically to secondary aldimines (i.e. R-CH=NR' where R' ≠ H).

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way due to historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states. For this reason salen-type compounds are used as metal deactivators. Metal salen complexes also find use as catalysts.
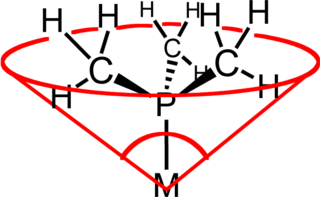
The ligand cone angle is a measure of the steric bulk of a ligand in a transition metal complex. It is defined as the solid angle formed with the metal at the vertex and the outermost edge of the van der Waals spheres of the ligand atoms at the perimeter of the cone. Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any ligand. The term cone angle was first introduced by Chadwick A. Tolman, a research chemist at DuPont. Tolman originally developed the method for phosphine ligands in nickel complexes, determining them from measurements of accurate physical models.
The Irving–Williams Series refers to the relative stabilities of complexes formed by transition metals. In 1953 Harry Irving and Robert Williams observed that the stability of complexes formed by divalent first-row transition metal ions generally increase across the period to a maximum stability at copper: Mn(II) < Fe(II) < Co(II) < Ni(II) < Cu(II) > Zn(II).

Decamethyldizincocene is an organozinc compound with the formula [Zn2(η5–C5Me5)2]. It is the first and an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water. It is stable at room temperature and especially soluble in diethyl ether, benzene, pentane, or tetrahydrofuran.

In coordination chemistry the bite angle is the ligand–metal–ligand bond angle of coordination complex containing a bidentate ligand. This geometric parameter is used to classify chelating ligands, including those in organometallic complexes. It is most often discussed in terms of catalysis, as changes in bite angle can affect not just the activity and selectivity of a catalytic reaction but even allow alternative reaction pathways to become accessible.
Methionine aminopeptidase 2 is an enzyme that in humans is encoded by the METAP2 gene.
A stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.

Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.
Enantioselective ketone reductions convert prochiral ketones into chiral, non-racemic alcohols and are used heavily for the synthesis of stereodefined alcohols.
Benzylic activation and stereocontrol in tricarbonyl(arene)chromium complexes refers to the enhanced rates and stereoselectivities of reactions at the benzylic position of aromatic rings complexed to chromium(0) relative to uncomplexed arenes. Complexation of an aromatic ring to chromium stabilizes both anions and cations at the benzylic position and provides a steric blocking element for diastereoselective functionalization of the benzylic position. A large number of stereoselective methods for benzylic and homobenzylic functionalization have been developed based on this property.
Tris(tert-butoxy)silanethiol is a silicon compound containing three tert-butoxy groups and a rare Si–S–H functional group. This colourless compound serves as an hydrogen donor in radical chain reactions. It was first prepared by alcoholysis of silicon disulfide and purified by distillation:

Reed McNeil Izatt is a Charles E. Maw Professor of Chemistry, Emeritus, at Brigham Young University in Provo, Utah. His field of research was macrocyclic chemistry and metal separation technologies.
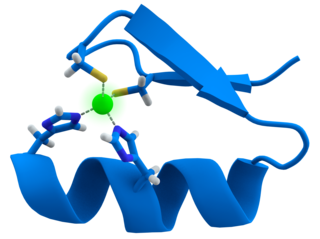
Transition metal thiolate complexes are metal complexes containing thiolate ligands. Thiolates are ligands that can be classified as soft Lewis bases. Therefore, thiolate ligands coordinate most strongly to metals that behave as soft Lewis acids as opposed to those that behave as hard Lewis acids. Most complexes contain other ligands in addition to thiolate, but many homoleptic complexes are known with only thiolate ligands. The amino acid cysteine has a thiol functional group, consequently many cofactors in proteins and enzymes feature cysteinate-metal cofactors.
Potassium octachlorodirhenate(III) is an inorganic compound with the formula K2Re2Cl8. This dark blue salt is well known as an early example of a compound featuring quadruple bond between its metal centers. Although the compound has no practical value, its characterization was significant in opening a new field of research into complexes with quadruple bonds.
In coordination chemistry, a macrocyclic ligand is a macrocyclic ring having at least nine atoms and three or more donor sites that serve as ligands that can bind to a central metal ion. Crown ethers and porphyrins are prominent examples. Macrocyclic ligands exhibit high affinity for metal ions.
In homogeneous catalysis, C2-symmetric ligands refer to ligands that lack mirror symmetry but have C2 symmetry. Such ligands are usually bidentate and are valuable in catalysis. The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. C2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including C2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.
Heterobimetallic catalysis is an approach to catalysis that employs two different metals to promote a chemical reaction. Included in this definition are cases where: 1) each metal activates a different substrate, 2) both metals interact with the same substrate, and 3) only one metal directly interacts with the substrate(s), while the second metal interacts with the first.

Metal cluster compounds are a molecular ion or neutral compound composed of three or more metals and featuring significant metal-metal interactions.


![Dihedral angle of [M(TC-m,n)] complex. Note that tropocoronand zinc complexes exhibit significantly larger dihedral angles relative to their Cu analogs, which is likely a result of the larger metal ionic radius. Dihedral angle TC complex 3.png](http://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Dihedral_angle_TC_complex_3.png/330px-Dihedral_angle_TC_complex_3.png)











