Related Research Articles

A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates, neutron-degenerate matter, and quark–gluon plasma. For a complete list of all exotic states of matter, see the list of states of matter.
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.
In physics, quasiparticles and collective excitations are closely related emergent phenomena arising when a microscopically complicated system such as a solid behaves as if it contained different weakly interacting particles in vacuum.
A thin film is a layer of material ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films is a fundamental step in many applications. A familiar example is the household mirror, which typically has a thin metal coating on the back of a sheet of glass to form a reflective interface. The process of silvering was once commonly used to produce mirrors, while more recently the metal layer is deposited using techniques such as sputtering. Advances in thin film deposition techniques during the 20th century have enabled a wide range of technological breakthroughs in areas such as magnetic recording media, electronic semiconductor devices, Integrated passive devices, LEDs, optical coatings, hard coatings on cutting tools, and for both energy generation and storage. It is also being applied to pharmaceuticals, via thin-film drug delivery. A stack of thin films is called a multilayer.
The molar heat capacity of a chemical substance is the amount of energy that must be added, in the form of heat, to one mole of the substance in order to cause an increase of one unit in its temperature. Alternatively, it is the heat capacity of a sample of the substance divided by the amount of substance of the sample; or also the specific heat capacity of the substance times its molar mass. The SI unit of molar heat capacity is joule per kelvin per mole, J⋅K−1⋅mol−1.
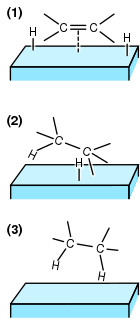
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
In physics, an entropic force acting in a system is an emergent phenomenon resulting from the entire system's statistical tendency to increase its entropy, rather than from a particular underlying force on the atomic scale.
Non-thermal microwave effects or specific microwave effects have been posited in order to explain unusual observations in microwave chemistry. The main effect of the absorption of microwaves by most materials is heating; the random motion of the constituent molecules is increased. Non-thermal effects are effects that are not due to the increase of thermal energy of the material. Instead, the microwave energy itself directly couples to energy modes within the molecule or lattice. Non-thermal effects in liquids are almost certainly non-existent, as the time for energy redistribution between molecules in a liquid is much less than the period of a microwave oscillation. A 2005 review has illustrated this in application to organic chemistry, though clearly supports the existence of non-thermal effects. It has been shown that such non-thermal effects exist in the reaction of O + HCl(DCl) -> OH(OD) + Cl in the gas phase and the authors suggest that some mechanisms may also be present in the condensed phase. Non-thermal effects in solids are still part of an ongoing debate. It is likely that through focusing of electric fields at particle interfaces, microwaves cause plasma formation and enhance diffusion in solids via second-order effects. As a result, they may enhance solid-state sintering processes. Debates continued in 2006 about non-thermal effects of microwaves that have been reported in solid-state phase transitions. A 2013 essay concluded the effect did not exist in organic synthesis involving liquid phases. A 2015 perspective discusses the non-thermal microwave effect in relation to selective heating by Debye relaxation processes.
Gas kinetics is a science in the branch of fluid dynamics, concerned with the study of motion of gases and its effects on physical systems. Based on the principles of fluid mechanics and thermodynamics, gas dynamics arises from the studies of gas flows in transonic and supersonic flights. To distinguish itself from other sciences in fluid dynamics, the studies in gas dynamics are often defined with gases flowing around or within physical objects at speeds comparable to or exceed the speed of sound and causing a significant change in temperature and pressure. Some examples of these studies include but are not limited to: choked flows in nozzles and valves, shock waves around jets, aerodynamic heating on atmospheric reentry vehicles and flows of gas fuel within a jet engine. At the molecular level, gas dynamics is a study of the kinetic theory of gases, often leading to the study of gas diffusion, statistical mechanics, chemical thermodynamics and non-equilibrium thermodynamics. Gas dynamics is synonymous with aerodynamics when the gas field is air and the subject of study is flight. It is highly relevant in the design of aircraft and spacecraft and their respective propulsion systems.

Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups Cs, D3h, and C2v. The C2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Carbon trioxide should not be confused with the stable carbonate ion (CO2−
3).
A two-dimensional gas is a collection of objects constrained to move in a planar or other two-dimensional space in a gaseous state. The objects can be: classical ideal gas elements such as rigid disks undergoing elastic collisions; elementary particles, or any ensemble of individual objects in physics which obeys laws of motion without binding interactions. The concept of a two-dimensional gas is used either because:
- the issue being studied actually takes place in two dimensions ; or,
- the two-dimensional form of the problem is more tractable than the analogous mathematically more complex three-dimensional problem.
Thermophoresis is a phenomenon observed in mixtures of mobile particles where the different particle types exhibit different responses to the force of a temperature gradient. This phenomenon tends to move light molecules to hot regions and heavy molecules to cold regions. The term thermophoresis most often applies to aerosol mixtures whose mean free path is comparable to its characteristic length scale , but may also commonly refer to the phenomenon in all phases of matter. The term Soret effect normally applies to liquid mixtures, which behave according to different, less well-understood mechanisms than gaseous mixtures. Thermophoresis may not apply to thermomigration in solids, especially multi-phase alloys.

In chemistry, a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water. Many such clusters have been predicted by theoretical models (in silico), and some have been detected experimentally in various contexts such as ice, bulk liquid water, in the gas phase, in dilute mixtures with non-polar solvents, and as water of hydration in crystal lattices. The simplest example is the water dimer (H2O)2.
In crystallography, a disclination is a line defect in which rotational symmetry is violated. In analogy with dislocations in crystals, the term, disinclination, for liquid crystals first used by Frederick Charles Frank and since then has been modified to its current usage, disclination. It is a defect in the orientation of director whereas a dislocation is a defect in positional order.

Gas is one of the four fundamental states of matter.
The hexatic phase is a state of matter that is between the solid and the isotropic liquid phases in two dimensional systems of particles. It is characterized by two order parameters: a short-range positional and a quasi-long-range orientational (sixfold) order. More generally, a hexatic is any phase that contains sixfold orientational order, in analogy with the nematic phase.
In physics and chemistry, a degree of freedom is an independent physical parameter in the formal description of the state of a physical system. The set of all states of a system is known as the system's phase space, and the degrees of freedom of the system are the dimensions of the phase space.

Self-propelled particles (SPP), also referred to as self-driven particles, are terms used by physicists to describe autonomous agents, which convert energy from the environment into directed or persistent motion. Natural systems which have inspired the study and design of these particles include walking, swimming or flying animals. Other biological systems include bacteria, cells, algae and other micro-organisms. Generally, self-propelled particles often refer to artificial systems such as robots or specifically designed particles such as swimming Janus colloids, bimetallic nanorods, nanomotors and walking grains. In the case of directed propulsion, which is driven by a chemical gradient, this is referred to as chemotaxis, observed in biological systems, e.g. bacteria quorum sensing and ant pheromone detection, and in synthetic systems, e.g. enzyme molecule chemotaxis and enzyme powered hard and soft particles.
DMol3 is a commercial software package which uses density functional theory with a numerical radial function basis set to calculate the electronic properties of molecules, clusters, surfaces and crystalline solid materials from first principles. DMol3 can either use gas phase boundary conditions or 3D periodic boundary conditions for solids or simulations of lower-dimensional periodicity. It has also pioneered the use of the conductor-like screening model COSMO Solvation Model for quantum simulations of solvated molecules and recently of wetted surfaces. DMol3 permits geometry optimisation and saddle point search with and without geometry constraints, as well as calculation of a variety of derived properties of the electronic configuration. DMol3 development started in the early eighties with B. Delley then associated with A.J. Freeman and D.E. Ellis at Northwestern University. In 1989 DMol3 appeared as DMol, the first commercial density functional package for industrial use by Biosym Technologies now Accelrys. Delley's 1990 publication was cited more than 3000 times.
References
- ↑ Waldmann, T. (2010). "Substrate registry in disordered layers of large molecules". ChemPhysChem. 11 (7): 1513–1517. doi:10.1002/cphc.200901028. PMID 20397239.
- ↑ Thomas Waldmann; Jens Klein; Harry E. Hoster; R. Jürgen Behm (2012), "Stabilization of Large Adsorbates by Rotational Entropy: A Time-Resolved Variable-Temperature STM Study", ChemPhysChem, vol. 14, no. 1, pp. 162–169, doi:10.1002/cphc.201200531, PMID 23047526, S2CID 36848079