Related Research Articles

In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero, i.e., 0 K. Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which microscopic quantum-mechanical phenomena, particularly wavefunction interference, become apparent macroscopically. More generally, condensation refers to the appearance of macroscopic occupation of one or several states: for example, in BCS theory, a superconductor is a condensate of Cooper pairs. As such, condensation can be associated with phase transition, and the macroscopic occupation of the state is the order parameter.

Helium is a chemical element; it has symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the elements, and it does not have a melting point at standard pressures. It is the second-lightest and second most abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy of helium-4, with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, the vast majority of which was formed during the Big Bang. Large amounts of new helium are created by nuclear fusion of hydrogen in stars.
Superfluid helium-4 is the superfluid form of helium-4, an isotope of the element helium. A superfluid is a state of matter in which matter behaves like a fluid with zero viscosity. The substance, which resembles other liquids such as helium I, flows without friction past any surface, which allows it to continue to circulate over obstructions and through pores in containers which hold it, subject only to its own inertia.

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates and Fermionic condensates, neutron-degenerate matter, and quark–gluon plasma.
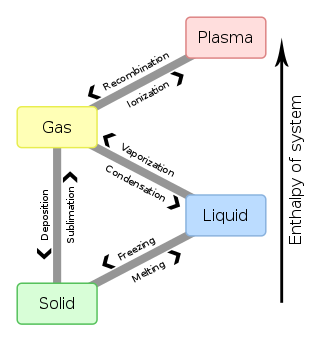
In physics, chemistry, and other related fields like biology, a phase transition is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point.

Viscount Ilya Romanovich Prigogine was a Belgian physical chemist of Russian-Jewish origin, noted for his work on dissipative structures, complex systems, and irreversibility.

Liquid helium is a physical state of helium at very low temperatures at standard atmospheric pressures. Liquid helium may show superfluidity.
Quantum turbulence is the name given to the turbulent flow – the chaotic motion of a fluid at high flow rates – of quantum fluids, such as superfluids. The idea that a form of turbulence might be possible in a superfluid via the quantized vortex lines was first suggested by Richard Feynman. The dynamics of quantum fluids are governed by quantum mechanics, rather than classical physics which govern classical (ordinary) fluids. Some examples of quantum fluids include superfluid helium, Bose–Einstein condensates (BECs), polariton condensates, and nuclear pasta theorized to exist inside neutron stars. Quantum fluids exist at temperatures below the critical temperature at which Bose-Einstein condensation takes place.

In condensed matter physics, a supersolid is a spatially ordered material with superfluid properties. In the case of helium-4, it has been conjectured since the 1960s that it might be possible to create a supersolid. Starting from 2017, a definitive proof for the existence of this state was provided by several experiments using atomic Bose–Einstein condensates. The general conditions required for supersolidity to emerge in a certain substance are a topic of ongoing research.

The lambda point is the temperature at which normal fluid helium makes the transition to superfluid state. At pressure of 1 atmosphere, the transition occurs at approximately 2.17 K. The lowest pressure at which He-I and He-II can coexist is the vapor−He-I−He-II triple point at 2.1768 K (−270.9732 °C) and 5.0418 kPa (0.049759 atm), which is the "saturated vapor pressure" at that temperature. The highest pressure at which He-I and He-II can coexist is the bcc−He-I−He-II triple point with a helium solid at 1.762 K (−271.388 °C), 29.725 atm (3,011.9 kPa).
John Frank Allen, FRS FRSE was a Canadian physicist. At the same time as Pyotr Leonidovich Kapitsa in Moscow, Don Misener and Allen independently discovered the superfluid phase of matter in 1937 using liquid helium in the Royal Society Mond Laboratory in Cambridge, England.

David Morris Lee is an American physicist who shared the 1996 Nobel Prize in Physics with Robert C. Richardson and Douglas Osheroff "for their discovery of superfluidity in helium-3." Lee is professor emeritus of physics at Cornell University and distinguished professor of physics at Texas A&M University.
A Rollin film, named after Bernard V. Rollin, is a 30 nm-thick liquid film of helium in the helium II state. It exhibits a "creeping" effect in response to surfaces extending past the film's level. Helium II can escape from any non-closed container via creeping toward and eventually evaporating from capillaries of 10 to 100 nm or greater.
Robert Herman was an American astronomer, best known for his work with Ralph Alpher in 1948–50, on estimating the temperature of cosmic microwave background radiation from the Big Bang explosion.
In fluid dynamics, inviscid flow is the flow of an inviscid fluid which is a fluid with zero viscosity.

In physics, a quantum vortex represents a quantized flux circulation of some physical quantity. In most cases, quantum vortices are a type of topological defect exhibited in superfluids and superconductors. The existence of quantum vortices was first predicted by Lars Onsager in 1949 in connection with superfluid helium. Onsager reasoned that quantisation of vorticity is a direct consequence of the existence of a superfluid order parameter as a spatially continuous wavefunction. Onsager also pointed out that quantum vortices describe the circulation of superfluid and conjectured that their excitations are responsible for superfluid phase transitions. These ideas of Onsager were further developed by Richard Feynman in 1955 and in 1957 were applied to describe the magnetic phase diagram of type-II superconductors by Alexei Alexeyevich Abrikosov. In 1935 Fritz London published a very closely related work on magnetic flux quantization in superconductors. London's fluxoid can also be viewed as a quantum vortex.
In condensed matter physics, second sound is a quantum mechanical phenomenon in which heat transfer occurs by wave-like motion, rather than by the more usual mechanism of diffusion. Its presence leads to a very high thermal conductivity. It is known as "second sound" because the wave motion of entropy and temperature is similar to the propagation of pressure waves in air (sound). The phenomenon of second sound was first described by Lev Landau in 1941.

Elephter Luarsabovich Andronikashvili was a Georgian physicist. He was a brother of Russian historian Irakly Andronikov.

Superfluidity is the characteristic property of a fluid with zero viscosity which therefore flows without any loss of kinetic energy. When stirred, a superfluid forms vortices that continue to rotate indefinitely. Superfluidity occurs in two isotopes of helium when they are liquefied by cooling to cryogenic temperatures. It is also a property of various other exotic states of matter theorized to exist in astrophysics, high-energy physics, and theories of quantum gravity. The theory of superfluidity was developed by Soviet theoretical physicists Lev Landau and Isaak Khalatnikov.

In the field of cryogenics, helium [He] is utilized for a variety of reasons. The combination of helium’s extremely low molecular weight and weak interatomic reactions yield interesting properties when helium is cooled below its critical temperature of 5.2 K to form a liquid. Even at absolute zero (0K), helium does not condense to form a solid under ambient pressure. In this state, the zero point vibrational energies of helium are comparable to very weak interatomic binding interactions, thus preventing lattice formation and giving helium its fluid characteristics. Within this liquid state, helium has two phases referred to as helium I and helium II. Helium I displays thermodynamic and hydrodynamic properties of classical fluids, along with quantum characteristics. However, below its lambda point of 2.17 K, helium transitions to He II and becomes a quantum superfluid with zero viscosity.
References
- ↑ Herman, Robert; Prigogine, Ilya (April 1979). "A Two-Fluid Approach to Town Traffic" (PDF). Science . 204 (4389): 148–151. Bibcode:1979Sci...204..148H. doi:10.1126/science.204.4389.148. PMID 17738075. S2CID 20780759. Archived from the original (PDF) on 2012-03-30.
- ↑ Khalatnikov, I.M. (2000). An Introduction to the Theory of Superfluidity. Westview Press. ISBN 978-0738203003.
- ↑ Balibar, S. (2017). "Laszlo Tisza and the two-fluid model of superfluidity". Comptes Rendus Physique. 18 (9–10): 586–591. doi:10.1016/j.crhy.2017.10.016.
- ↑ Barna, I.F.; Mátyás, L. (2021). "Analytic Solutions of a Two-Fluid Hydrodynamic Model". Mathematical Modelling and Analysis. 26 (4): 582–590. arXiv: 2004.09815 . doi:10.3846/mma.2021.13637.