
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current in a circuit is opposite to the direction of electron flow, so electrons flow from the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "+" is the cathode.
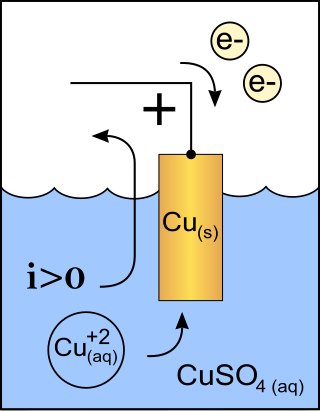
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic CCD for Cathode Current Departs. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic cathode current departs also means that electrons flow into the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode.

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. It was invented by Italian chemist Alessandro Volta, who published his experiments in 1799. Its invention can be traced back to an argument between Volta and Luigi Galvani, Volta's fellow Italian scientist who had conducted experiments on frogs' legs. Use of the voltaic pile enabled a rapid series of other discoveries, including the electrical decomposition (electrolysis) of water into oxygen and hydrogen by William Nicholson and Anthony Carlisle (1800), and the discovery or isolation of the chemical elements sodium (1807), potassium (1807), calcium (1808), boron (1808), barium (1808), strontium (1808), and magnesium (1808) by Humphry Davy.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."
In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrode (SHE). It is defined to have a potential of zero volts. It may also be defined as the potential difference between the charged metallic rods and salt solution.

Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated, and the anode is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.
The Hall–Héroult process is the major industrial process for smelting aluminium. It involves dissolving aluminium oxide (alumina) in molten cryolite and electrolyzing the molten salt bath, typically in a purpose-built cell. The Hall–Héroult process applied at industrial scale happens at 940–980 °C and produces 99.5–99.8% pure aluminium. Recycling aluminum requires no electrolysis, thus it is not treated in this way.

A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.
In electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. The IUPAC "Gold Book" defines it as; "the value of the standard emf of a cell in which molecular hydrogen under standard pressure is oxidized to solvated protons at the left-hand electrode".
In electrochemistry, a half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electric charges between the electrode and the electrolyte, resulting in a potential difference between the electrode and the electrolyte. The typical anode reaction involves a metal atom in the electrode being dissolved and transported as a positive ion across the double layer, causing the electrolyte to acquire a net positive charge while the electrode acquires a net negative charge. The growing potential difference creates an intense electric field within the double layer, and the potential rises in value until the field halts the net charge-pumping reactions. This self-limiting action occurs almost instantly in an isolated half-cell; in applications two dissimilar half-cells are appropriately connected to constitute a Galvanic cell.
Electropolishing, also known as electrochemical polishing, anodic polishing, or electrolytic polishing, is an electrochemical process that removes material from a metallic workpiece, reducing the surface roughness by levelling micro-peaks and valleys, improving the surface finish. Electropolishing is often compared to, but distinctly different from, electrochemical machining. It is used to polish, passivate, and deburr metal parts. It is often described as the reverse of electroplating. It may be used in lieu of abrasive fine polishing in microstructural preparation.

Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
Silicon–air batteries are a new battery technology invented by a team led by Prof. Ein-Eli at the Grand Technion Energy Program at the Technion – Israel Institute of Technology.

Bipolar electrochemistry is a phenomenon in electrochemistry based on the polarization of conducting objects in electric fields. Indeed, this polarization generates a potential difference between the two extremities of the substrate that is equal to the electric field value multiplied by the size of the object. If this potential difference is important enough, then redox reactions can be generated at the extremities of the object, oxidations will occur at one extremity coupled simultaneously to reductions at the other extremity. In a simple experimental setup consisting of a platinum wire in a weighing boat containing a pH indicator solution, a 30 V voltage across two electrodes will cause water reduction at one end of the wire and a pH increase and water oxidation at the anodic end and a pH decrease. The poles of the bipolar electrode also align themselves with the applied electric field.

Galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. This phenomenon is named after Italian physician Luigi Galvani (1737–1798).
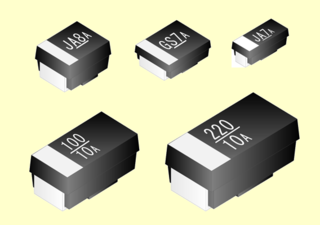
A niobium electrolytic capacitor is an electrolytic capacitor whose anode (+) is made of passivated niobium metal or niobium monoxide, on which an insulating niobium pentoxide layer acts as a dielectric. A solid electrolyte on the surface of the oxide layer serves as the capacitor's cathode (−).











