Chemical structures
Vibralactone A | Vibralactone B | Vibralactone C |
Vibralactone D | Vibralactone E | Vibralactone F |
Vibralactones are a group of related terpenoid chemical compounds, the first of which were isolated from the Basidiomycete Boreostereum vibrans in 2006. [1] Additional vibralactones have also been isolated from the fungus Stereum hirsutum . [2]
Vibralactone A has attracted research interest because it is an inhibitor of pancreatic lipase (IC50 0.4 μg/mL) with in vitro activity comparable to that of the obesity drug orlistat. [1] [3]
Several laboratory syntheses of vibralactone A have been developed. [3] [4]
Vibralactone A | Vibralactone B | Vibralactone C |
Vibralactone D | Vibralactone E | Vibralactone F |
Strobilurins are a group of natural products and their synthetic analogs. A number of strobilurins are used in agriculture as fungicides. They are part of the larger group of QoIs, which act to inhibit the respiratory chain at the level of Complex III.

Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth.

Hydrogen peroxide - urea is a solid composed of equal amounts of hydrogen peroxide and urea. This compound is a white crystalline solid which dissolves in water to give free hydrogen peroxide. Hydrogen peroxide - urea contains solid and water-free hydrogen peroxide, which offers a higher stability and better controllability than liquid hydrogen peroxide when used as an oxidizing agent. Often called carbamide peroxide in the dental office, it is used as a source of hydrogen peroxide for bleaching, disinfection, and oxidation.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

Stereum hirsutum, also called false turkey tail and hairy curtain crust, is a fungus typically forming multiple brackets on dead wood. It is also a plant pathogen infecting peach trees. S. hirsutum is in turn parasitised by certain other species such as the fungus Tremella aurantia. Substrates for S. hirsutum include dead limbs and trunks of both hardwoods and conifers.

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.
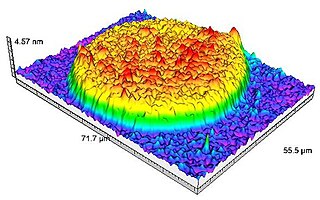
Sarfus is an optical quantitative imaging technique based on the association of:

Taxodone is a naturally occurring diterpenoid found in Taxodium distichum, Rosmarinus officinalis (rosemary), several salvia species and other plants, along with its oxidized rearrangement product, taxodione. Taxodone and taxodione exhibit anticancer, antibacterial, antioxidant, antifungal, insecticide, and antifeedant activities.

Spiruchostatins are a group of chemical compounds isolated from Pseudomonas sp. as gene expression-enhancing substances. They possess novel bicyclic depsipeptides involving 4-amino-3-hydroxy-5-methylhexanoic acid and 4-amino-3-hydroxy-5-methylheptanoic acid residues. The two main forms are spiruchostatin A and spiruchostatin B.

LY-2183240 is a drug which acts both as a potent inhibitor of the reuptake of the endocannabinoid anandamide and as an inhibitor of fatty acid amide hydrolase (FAAH), the primary enzyme responsible for degrading anandamide. This leads to markedly elevated anandamide levels in the brain, and LY-2183240 has been shown to produce both analgesic and anxiolytic effects in animal models. While LY-2183240 is a potent inhibitor of FAAH, it has relatively poor selectivity and also inhibits several other enzyme side targets. Consequently, it was never developed for clinical use, though it remains widely used in research, and has also been sold as a designer drug.
Boreostereum is a genus of corticioid fungi. The genus was circumscribed in 1968 by Erast Parmasto to contain the type species, which was formerly known as Stereum radiatum. Boreostereum has four species that are widely distributed in northern temperate areas. Species in the genus have a dimitic hyphal system, and the hyphae have with brown encrustations that turn greenish when potassium hydroxide is applied. Boreosterum vibrans produces vibralactones, chemical metabolites that inhibit various enzymes. Recent phylogenetic research indicates that Boreostereum is a sister group to the rest of the Gloeophyllales.
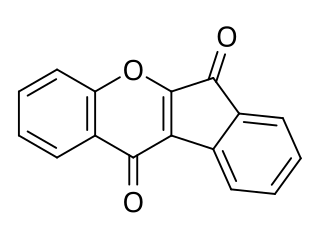
Wrightiadione is an isoflavone that occurs in the plant Wrightia tomentosa and can also be synthesized. It is a novel template for the TrkA kinase inhibitors.

Lipase inhibitors belong to a drug class that is used as an antiobesity agent. Their mode of action is to inhibit gastric and pancreatic lipases, enzymes that play an important role in the digestion of dietary fat. Lipase inhibitors are classified in the ATC-classification system as A08AB . Numerous compounds have been either isolated from nature, semi-synthesized, or fully synthesized and then screened for their lipase inhibitory activity but the only lipase inhibitor on the market is orlistat . Lipase inhibitors have also shown anticancer activity, by inhibiting fatty acid synthase.
Mark S. Cushman is an American chemist, whose primary research is in the area of medicinal chemistry. He completed his pre-pharmacy studies at Fresno State College (now California State University, Fresno) in 1965. He then attended the University of California San Francisco (as a University of California Regents Scholar), earning a Pharm.D. in 1969 and a Ph.D. in Medicinal Chemistry in 1973. Thereafter, he performed postdoctoral training in the laboratory of George Büchi, Ph.D., at the Massachusetts Institute of Technology (MIT). There, his research focused on the discovery and development of new synthetic methodologies, and the isolation and structural characterization of mycotoxins from Aspergillus niger. In 1975, he joined the Department of Medicinal Chemistry and Molecular Pharmacology (at the time, Department of Medicinal Chemistry and Pharmacognosy) at Purdue University. From 1983 to 1984, Prof. Cushman was a Senior Fulbright Scholar at Munich Technical University working in the laboratory of Professor Adelbert Bacher. His sabbatical work dealt with the design and synthesis of probes to elucidate key aspects of the biosynthesis of riboflavin (vitamin B2). Currently he holds the rank of Distinguished Professor Emeritus of Medicinal Chemistry at Purdue University. He has mentored 40 graduate students, 59 postdoctoral researchers, and 5 visiting scholars. He has published 348 papers and holds 41 patents. His work has ~17,000 citations with an h-index of 69. His most cited papers had 471, 403, and 299 citations as of August 2021. He has made seminal contributions to the fields of synthetic and medicinal chemistry including the development of new synthetic methodologies, the synthesis of natural products, and the preparation of antivirals, antibacterials, and anticancer agents, and mechanism probes to understand the function of over thirty macromolecular targets. One of his main scientific contributions is the development of the indenoisoquinolines, molecules that inhibit the action of toposiomerase I (Top1) and stabilize the G-quadruplex in the Myc promoter. Three indenoisoquinolines designed and synthesized by his research group at Purdue University [indotecan (LMP 400), indimitecan (LMP 776), and LMP 744] demonstrated potent anticancer activity in vivo and have completed phase I clinical trials at the National Institutes of Health.
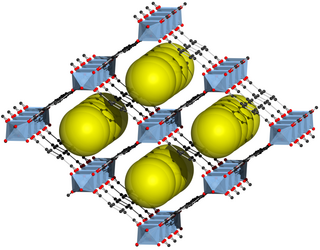
MIL-53 belongs to the class of metal-organic framework (MOF) materials. The first synthesis and the name was established by the group of Gérard Férey in 2002. The MIL-53 structure consists of inorganic [M-OH] chains, which are connected to four neighboring inorganic chains by therephthalate-based linker molecules. Each metal center is octahedrally coordinated by six oxygen atoms. Four of these oxygen atoms originate from four different carboxylate groups and the remaining two oxygen atoms belong to two different μ-OH moieties, which bridge neighboring metal centers. The resulting framework structure contains one-dimensional diamond-shaped pores. Many research group have investigated the flexibility of the MIL-53 structure. This flexible behavior, during which the pore cross-section changes reversibly, was termed 'breathing.effect' and describes the ability of the MIL-53 framework to respond to external stimuli.

Bruceantin is a chemical compound that was first isolated from the plant Brucea antidysenterica in 1973. Chemically, it is classified as a secotriterpenoid and a quassinoid.

1,4-Pentadiyne (penta-1,4-diyne) is a chemical compound belonging to the alkynes. The compound is the structural isomer to 1,3-pentadiyne.
{{cite journal}}: CS1 maint: multiple names: authors list (link){{cite journal}}: CS1 maint: multiple names: authors list (link){{cite journal}}: CS1 maint: multiple names: authors list (link){{cite journal}}: CS1 maint: multiple names: authors list (link)