
The visual cortex of the brain is the area of the cerebral cortex that processes visual information. It is located in the occipital lobe. Sensory input originating from the eyes travels through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. The area of the visual cortex that receives the sensory input from the lateral geniculate nucleus is the primary visual cortex, also known as visual area 1 (V1), Brodmann area 17, or the striate cortex. The extrastriate areas consist of visual areas 2, 3, 4, and 5.
Neural adaptation or sensory adaptation is a gradual decrease over time in the responsiveness of the sensory system to a constant stimulus. It is usually experienced as a change in the stimulus. For example, if a hand is rested on a table, the table's surface is immediately felt against the skin. Subsequently, however, the sensation of the table surface against the skin gradually diminishes until it is virtually unnoticeable. The sensory neurons that initially respond are no longer stimulated to respond; this is an example of neural adaptation.
Microsaccades are a kind of fixational eye movement. They are small, jerk-like, involuntary eye movements, similar to miniature versions of voluntary saccades. They typically occur during prolonged visual fixation, not only in humans, but also in animals with foveal vision. Microsaccade amplitudes vary from 2 to 120 arcminutes. The first empirical evidence for their existence was provided by Robert Darwin, the father of Charles Darwin.
The concept of backward masking originated in psychoacoustics, referring to temporal masking of quiet sounds that occur moments before a louder sound.
Salience is the property by which some thing stands out. Salient events are an attentional mechanism by which organisms learn and survive; those organisms can focus their limited perceptual and cognitive resources on the pertinent subset of the sensory data available to them.
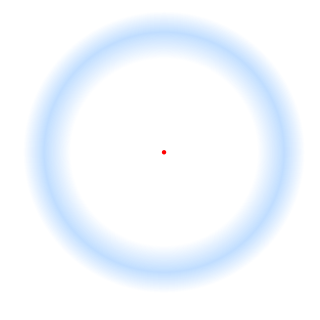
In vision, filling-in phenomena are those responsible for the completion of missing information across the physiological blind spot, and across natural and artificial scotomata. There is also evidence for similar mechanisms of completion in normal visual analysis. Classical demonstrations of perceptual filling-in involve filling in at the blind spot in monocular vision, and images stabilized on the retina either by means of special lenses, or under certain conditions of steady fixation. For example, naturally in monocular vision at the physiological blind spot, the percept is not a hole in the visual field, but the content is “filled-in” based on information from the surrounding visual field. When a textured stimulus is presented centered on but extending beyond the region of the blind spot, a continuous texture is perceived. This partially inferred percept is paradoxically considered more reliable than a percept based on external input..
Neural coding is a neuroscience field concerned with characterising the hypothetical relationship between the stimulus and the neuronal responses, and the relationship among the electrical activities of the neurons in the ensemble. Based on the theory that sensory and other information is represented in the brain by networks of neurons, it is believed that neurons can encode both digital and analog information.

The Chubb illusion is an optical illusion or error in visual perception in which the apparent contrast of an object varies substantially to most viewers depending on its relative contrast to the field on which it is displayed. These visual illusions are of particular interest to researchers because they may provide valuable insights in regard to the workings of human visual systems.
Repetition priming refers to improvements in a behavioural response when stimuli are repeatedly presented. The improvements can be measured in terms of accuracy or reaction time and can occur when the repeated stimuli are either identical or similar to previous stimuli. These improvements have been shown to be cumulative, so as the number of repetitions increases the responses get continually faster up to a maximum of around seven repetitions. These improvements are also found when the repeated items are changed slightly in terms of orientation, size and position. The size of the effect is also modulated by the length of time the item is presented for and the length time between the first and subsequent presentations of the repeated items.

Fixation or visual fixation is the maintaining of the gaze on a single location. An animal can exhibit visual fixation if it possess a fovea in the anatomy of their eye. The fovea is typically located at the center of the retina and is the point of clearest vision. The species in which fixational eye movement has been verified thus far include humans, primates, cats, rabbits, turtles, salamanders, and owls. Regular eye movement alternates between saccades and visual fixations, the notable exception being in smooth pursuit, controlled by a different neural substrate that appears to have developed for hunting prey. The term "fixation" can either be used to refer to the point in time and space of focus or the act of fixating. Fixation, in the act of fixating, is the point between any two saccades, during which the eyes are relatively stationary and virtually all visual input occurs. In the absence of retinal jitter, a laboratory condition known as retinal stabilization, perceptions tend to rapidly fade away. To maintain visibility, the nervous system carries out a procedure called fixational eye movement, which continuously stimulates neurons in the early visual areas of the brain responding to transient stimuli. There are three categories of fixational eye movement: microsaccades, ocular drifts, and ocular microtremor. At small amplitudes the boundaries between categories become unclear, particularly between drift and tremor.
Feature detection is a process by which the nervous system sorts or filters complex natural stimuli in order to extract behaviorally relevant cues that have a high probability of being associated with important objects or organisms in their environment, as opposed to irrelevant background or noise.
In the psychology of perception and motor control, the term response priming denotes a special form of priming. Generally, priming effects take place whenever a response to a target stimulus is influenced by a prime stimulus presented at an earlier time. The distinctive feature of response priming is that prime and target are presented in quick succession and are coupled to identical or alternative motor responses. When a speeded motor response is performed to classify the target stimulus, a prime immediately preceding the target can thus induce response conflicts when assigned to a different response as the target. These response conflicts have observable effects on motor behavior, leading to priming effects, e.g., in response times and error rates. A special property of response priming is its independence from visual awareness of the prime.
Subliminal stimuli are any sensory stimuli below an individual's threshold for conscious perception, in contrast to supraliminal stimuli.

Due to the effect of a spatial context or temporal context, the perceived orientation of a test line or grating pattern can appear tilted away from its physical orientation. The tilt illusion (TI) is the phenomenon that the perceived orientation of a test line or grating is altered by the presence of surrounding lines or grating with a different orientation. And the tilt aftereffect (TAE) is the phenomenon that the perceived orientation is changed after prolonged inspection of another oriented line or grating.

Visual processing abnormalities in schizophrenia are commonly found, and contribute to poor social function.
Many experiments have been done to find out how the brain interprets stimuli and how animals develop fear responses. The emotion, fear, has been hard-wired into almost every individual, due to its vital role in the survival of the individual. Researchers have found that fear is established unconsciously and that the amygdala is involved with fear conditioning.
Binocular neurons are neurons in the visual system that assist in the creation of stereopsis from binocular disparity. They have been found in the primary visual cortex where the initial stage of binocular convergence begins. Binocular neurons receive inputs from both the right and left eyes and integrate the signals together to create a perception of depth.
Surround suppression is where the relative firing rate of a neuron may under certain conditions decrease when a particular stimulus is enlarged. It has been observed in electrophysiology studies of the brain and has been noted in many sensory neurons, most notably in the early visual system. Surround suppression is defined as a reduction in the activity of a neuron in response to a stimulus outside its classical receptive field.

Stephen Louis Macknik is an American neuroscientist and science writer. He is a Professor of Ophthalmology, Neurology, and Physiology & Pharmacology at the State University of New York, Downstate Medical Center, where he directs the Laboratory of Translational Neuroscience. He directed laboratories previously at the Barrow Neurological Institute and University College London. He is best known for his studies on illusions, consciousness, attentional misdirection in stage magic, and cerebral blood flow.
Binocular switch suppression (BSS) is a technique to suppress usually salient images from an individual's awareness, a type of experimental manipulation used in visual perception and cognitive neuroscience. In BSS, two images of differing signal strengths are repetitively switched between the left and right eye at a constant rate of 1 Hertz. During this process of switching, the image of lower contrast and signal strength is perceptually suppressed for a period of time.






