
Pharmaceutical Research and Manufacturers of America, formerly known as the Pharmaceutical Manufacturers Association, is a trade group representing companies in the pharmaceutical industry in the United States. Founded in 1958, PhRMA lobbies on behalf of pharmaceutical companies. PhRMA is headquartered in Washington, DC.

Sydney Grammar School is an independent, fee-paying, non-denominational day school for boys, located in Sydney, Australia.

Gilead Sciences, Inc. is an American biopharmaceutical company headquartered in Foster City, California that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500.
Bausch Health Companies Inc. is an American-Canadian multinational specialty pharmaceutical company based in Laval, Quebec, Canada. It develops, manufactures and markets pharmaceutical products and branded generic drugs, primarily for skin diseases, gastrointestinal disorders, eye health and neurology. Bausch Health owns Bausch & Lomb, a supplier of eye health products. Bausch Health's business model is primarily focused on acquiring small pharmaceutical companies and then sharply increasing the prices of the drugs these companies sell.

Pyrimethamine, sold under the brand name Daraprim among others, is a medication used with leucovorin to treat the parasitic diseases toxoplasmosis and cystoisosporiasis. It is also used with dapsone as a second-line option to prevent Pneumocystis jiroveci pneumonia in people with HIV/AIDS. It was previously used for malaria but is no longer recommended due to resistance. Pyrimethamine is taken by mouth.
Epilepsy in animals is a group of neurological disorders characterized by seizures, caused by uncontrolled, abnormal bursts of electrical activity in the brain. They can start and stop very abruptly and last any amount of time from a few seconds to a few minutes. Canine epilepsy is often genetic but epilepsy in cats and other pets is rarer, likely because there is no hereditary component to epilepsy in these animals.

Mecamylamine is a non-selective, non-competitive antagonist of the nicotinic acetylcholine receptors (nAChRs) that was introduced in the 1950s as an antihypertensive drug. In the United States, it was voluntarily withdrawn from the market in 2009 but was brought to market in 2013 as Vecamyl and eventually was marketed by Turing Pharmaceuticals.
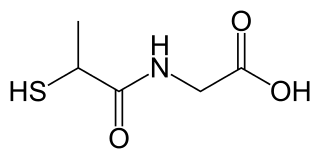
Tiopronin, sold under the brand name Thiola, is a medication used to control the rate of cystine precipitation and excretion in the disease cystinuria.
Medication costs, also known as drug costs are a common health care cost for many people and health care systems. Prescription costs are the costs to the end consumer. Medication costs are influenced by multiple factors such as patents, stakeholder influence, and marketing expenses. A number of countries including Canada, parts of Europe, and Brazil use external reference pricing as a means to compare drug prices and to determine a base price for a particular medication. Other countries use pharmacoeconomics, which looks at the cost/benefit of a product in terms of quality of life, alternative treatments, and cost reduction or avoidance in other parts of the health care system. Structures like the UK's National Institute for Health and Clinical Excellence and to a lesser extent Canada's Common Drug Review evaluate products in this way.
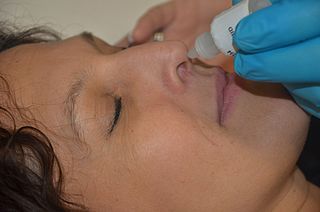
Nasal administration, popularly known as snorting, is a route of administration in which drugs are insufflated through the nose. It can be a form of either topical administration or systemic administration, as the drugs thus locally delivered can go on to have either purely local or systemic effects ibuprofen or Tylenol for headaches along with pains such as severe toothaches. Nasal sprays are locally acting drugs such as decongestants for cold and allergy treatment, whose systemic effects are usually minimal. Examples of systemically active drugs available as nasal sprays are migraine drugs, rescue medications for overdose and seizure emergencies, hormone treatments, nicotine nasal spray, and nasal vaccines such as live attenuated influenza vaccine.
Sulfadoxine/pyrimethamine, sold under the brand name Fansidar, is a combination medication used to treat malaria. It contains sulfadoxine and pyrimethamine. For the treatment of malaria it is typically used along with other antimalarial medication such as artesunate. In areas of Africa with moderate to high rates of malaria, three doses are recommended during the second and third trimester of pregnancy.

Martin Shkreli is an American investor and businessman. He was convicted of financial crimes for which he served over six years in federal prison and was fined over 70 million dollars. Shkreli is the co-founder of the hedge funds Elea Capital, MSMB Capital Management, and MSMB Healthcare, the co-founder and former CEO of pharmaceutical firms Retrophin and Turing Pharmaceuticals, and the former CEO of start-up software company Gödel Systems, which he founded in August 2016.
Specialty drugs or specialty pharmaceuticals are a recent designation of pharmaceuticals classified as high-cost, high complexity and/or high touch. Specialty drugs are often biologics—"drugs derived from living cells" that are injectable or infused. They are used to treat complex or rare chronic conditions such as cancer, rheumatoid arthritis, hemophilia, H.I.V. psoriasis, inflammatory bowel disease and hepatitis C. In 1990 there were 10 specialty drugs on the market, around five years later nearly 30, by 2008 200, and by 2015 300.
Harrow Health, Inc., formerly known as Imprimis Pharmaceuticals, is a publicly traded pharmaceutical company based in Nashville, Tennessee.
Tonix Pharmaceuticals is a pharmaceutical company based in Chatham, New Jersey that focuses on repurposed drugs for central nervous system conditions and as of 2020 was also pursuing a vaccine for COVID-19 and a biodefense project.
Access to medicines refers to the reasonable ability for people to get needed medicines required to achieve health. Such access is deemed to be part of the right to health as supported by international law since 1946.

The Four Thieves Vinegar Collective is an anarchist biohacking group founded in 2015 by Michael Laufer. They have published instructions for the "EpiPencil", an epinephrine autoinjector, and the "Apothecary MicroLab", a do-it-yourself (DIY) device intended to make a variety of medications, most notably pyrimethamine (Daraprim). The medical community has criticized them for causing potential harm to patients with the DIY instructions, but Laufer claims to defend people's right to attempt their medical treatment.
The Pharmaceutical Accountability Foundation was established in Amsterdam in July 2018 to deal with pharmaceutical companies that demand excessive prices for medicines in the Netherlands. This followed a report by the Raad voor de Volksgezondheid en Zorg in 2017. Wilbert Bannenberg, an epidemiologist, is the chairman. The group plans to deploy both health and pharmaceutical expertise and lawyers.

Alice Elizabeth Motion is a British chemist, science communicator, and associate professor at the School of Chemistry, University of Sydney. She is the founder of the Breaking Good project which encourages high school and undergraduate students to take part in research that can benefit human health. In 2018, the Breaking Good project was a finalist on the Google.org Impact Challenge.

Matthew Houghton Todd is a British chemist and the Professor and Chair of Drug Discovery of the School of Pharmacy at University College London. He is the founder of Open Source Malaria (OSM) and his research focuses on drug discovery and development for this disease. Recently, he has expanded to other areas, particularly neglected diseases such as tuberculosis and mycetoma in the Open Source Tuberculosis (OSTB) and Open Source Mycetoma (MycetOS) project, through a collaboration with the Drugs for Neglected Diseases Initiative and Erasmus MC. In addition, he has some research activity in catalysis and methodology.











