
Rats are various medium-sized, long-tailed rodents. Species of rats are found throughout the order Rodentia, but stereotypical rats are found in the genus Rattus. Other rat genera include Neotoma, Bandicota and Dipodomys.

A saccade is a quick, simultaneous movement of both eyes between two or more phases of fixation in the same direction. In contrast, in smooth pursuit movements, the eyes move smoothly instead of in jumps. The phenomenon can be associated with a shift in frequency of an emitted signal or a movement of a body part or device. Controlled cortically by the frontal eye fields (FEF), or subcortically by the superior colliculus, saccades serve as a mechanism for fixation, rapid eye movement, and the fast phase of optokinetic nystagmus. The word appears to have been coined in the 1880s by French ophthalmologist Émile Javal, who used a mirror on one side of a page to observe eye movement in silent reading, and found that it involves a succession of discontinuous individual movements.

Whiskers or vibrissae are a type of mammalian hair that are typically characterised, anatomically, by their long length, large and well-innervated hair follicle, and by having an identifiable representation in the somatosensory cortex of the brain.

The claustrum is a thin, bilateral structure that connects to cortical and subcortical regions of the brain. It is located between the insula laterally and the putamen medially, separated by the extreme and external capsules respectively. The blood supply to the claustrum is fulfilled via the middle cerebral artery. It is considered to be the most densely connected structure in the brain, allowing for integration of various cortical inputs into one experience rather than singular events. The claustrum is difficult to study given the limited number of individuals with claustral lesions and the poor resolution of neuroimaging.

The motor cortex is the region of the cerebral cortex involved in the planning, control, and execution of voluntary movements. Classically, the motor cortex is an area of the frontal lobe located in the posterior precentral gyrus immediately anterior to the central sulcus.
In physiology, tonotopy is the spatial arrangement of where sounds of different frequency are processed in the brain. Tones close to each other in terms of frequency are represented in topologically neighbouring regions in the brain. Tonotopic maps are a particular case of topographic organization, similar to retinotopy in the visual system.

The barrel cortex is a region of the somatosensory cortex that is identifiable in some species of rodents and species of at least two other orders and contains the barrel field. The 'barrels' of the barrel field are regions within cortical layer IV that are visibly darker when stained to reveal the presence of cytochrome c oxidase and are separated from each other by lighter areas called septa. These dark-staining regions are a major target for somatosensory inputs from the thalamus, and each barrel corresponds to a region of the body. Due to this distinctive cellular structure, organisation, and functional significance, the barrel cortex is a useful tool to understand cortical processing and has played an important role in neuroscience. The majority of what is known about corticothalamic processing comes from studying the barrel cortex, and researchers have intensively studied the barrel cortex as a model of neocortical column.

The stria terminalis is a structure in the brain consisting of a band of fibers running along the lateral margin of the ventricular surface of the thalamus. Serving as a major output pathway of the amygdala, the stria terminalis runs from its centromedial division to the ventromedial nucleus of the hypothalamus.
Head direction (HD) cells are neurons found in a number of brain regions that increase their firing rates above baseline levels only when the animal's head points in a specific direction. They have been reported in rats, monkeys, mice, chinchillas and bats, but are thought to be common to all mammals, perhaps all vertebrates and perhaps even some invertebrates, and to underlie the "sense of direction". When the animal's head is facing in the cell's "preferred firing direction" these neurons fire at a steady rate, but firing decreases back to baseline rates as the animal's head turns away from the preferred direction.
Theta waves generate the theta rhythm, a neural oscillatory pattern that can be seen on an electroencephalogram (EEG), recorded either from inside the brain or from electrodes attached to the scalp. Two types of theta rhythm have been described. The hippocampal theta rhythm is a strong oscillation that can be observed in the hippocampus and other brain structures in numerous species of mammals including rodents, rabbits, dogs, cats, bats, and marsupials. "Cortical theta rhythms" are low-frequency components of scalp EEG, usually recorded from humans. Theta rhythms can be quantified using quantitative electroencephalography (qEEG) using freely available toolboxes, such as, EEGLAB or the Neurophysiological Biomarker Toolbox (NBT).

The frontal eye fields (FEF) are a region located in the frontal cortex, more specifically in Brodmann area 8 or BA8, of the primate brain. In humans, it can be more accurately said to lie in a region around the intersection of the middle frontal gyrus with the precentral gyrus, consisting of a frontal and parietal portion. The FEF is responsible for saccadic eye movements for the purpose of visual field perception and awareness, as well as for voluntary eye movement. The FEF communicates with extraocular muscles indirectly via the paramedian pontine reticular formation. Destruction of the FEF causes deviation of the eyes to the ipsilateral side.

Cat senses are adaptations that allow cats to be highly efficient predators. Cats are good at detecting movement in low light, have an acute sense of hearing and smell, and their sense of touch is enhanced by long whiskers that protrude from their heads and bodies. These senses evolved to allow cats to hunt effectively at night.

The tawny-bellied cotton rat is a species of rodent in the family Cricetidae. It is found in Mexico and in the US states of Arizona and New Mexico.
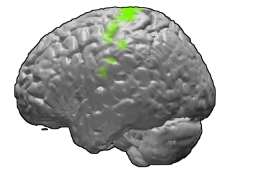
The primary motor cortex is a brain region that in humans is located in the dorsal portion of the frontal lobe. It is the primary region of the motor system and works in association with other motor areas including premotor cortex, the supplementary motor area, posterior parietal cortex, and several subcortical brain regions, to plan and execute movements. Primary motor cortex is defined anatomically as the region of cortex that contains large neurons known as Betz cells. Betz cells, along with other cortical neurons, send long axons down the spinal cord to synapse onto the interneuron circuitry of the spinal cord and also directly onto the alpha motor neurons in the spinal cord which connect to the muscles.
Levodopa-induced dyskinesia (LID) is a form of dyskinesia associated with levodopa (l-DOPA), used to treat Parkinson's disease. It often involves hyperkinetic movements, including chorea, dystonia, and athetosis.

Hydrodynamic reception refers to the ability of some animals to sense water movements generated by biotic or abiotic sources. This form of mechanoreception is useful for orientation, hunting, predator avoidance, and schooling. Frequent encounters with conditions of low visibility can prevent vision from being a reliable information source for navigation and sensing objects or organisms in the environment. Sensing water movements is one resolution to this problem.
Sniffing is a perceptually-relevant behavior, defined as the active sampling of odors through the nasal cavity for the purpose of information acquisition. This behavior, displayed by all terrestrial vertebrates, is typically identified based upon changes in respiratory frequency and/or amplitude, and is often studied in the context of odor guided behaviors and olfactory perceptual tasks. Sniffing is quantified by measuring intra-nasal pressure or flow or air or, while less accurate, through a strain gauge on the chest to measure total respiratory volume. Strategies for sniffing behavior vary depending upon the animal, with small animals displaying sniffing frequencies ranging from 4 to 12 Hz but larger animals (humans) sniffing at much lower frequencies, usually less than 2 Hz. Subserving sniffing behaviors, evidence for an "olfactomotor" circuit in the brain exists, wherein perception or expectation of an odor can trigger brain respiratory center to allow for the modulation of sniffing frequency and amplitude and thus acquisition of odor information. Sniffing is analogous to other stimulus sampling behaviors, including visual saccades, active touch, and whisker movements in small animals. Atypical sniffing has been reported in cases of neurological disorders, especially those disorders characterized by impaired motor function and olfactory perception.
Sharp waves and ripples (SWRs) are oscillatory patterns in the mammalian brain hippocampus seen on an EEG during immobility and sleep. There are three major network oscillation patterns in the hippocampus: theta waves, SWRs and gamma waves. Gamma oscillations are found in all major brain structures, whereas theta and sharp waves are specific to the hippocampus and its neighbouring areas. SWRs are composed of large amplitude sharp waves in local field potential and associated fast field oscillations known as ripples. SWRs are shown to be involved in memory consolidation and the replay of wakefulness-acquired memory. These network oscillations are the most synchronous patterns in the brain, making them susceptible to pathological patterns such as epilepsy.

The grimace scale (GS), sometimes called the grimace score, is a method of assessing the occurrence or severity of pain experienced by non-human animals according to objective and blinded scoring of facial expressions, as is done routinely for the measurement of pain in non-verbal humans. Observers score the presence or prominence of “facial action units" (FAU), e.g. Orbital Tightening, Nose Bulge, Ear Position and Whisker Change. These are scored by observing the animal directly in real-time, or post hoc from photographs or screen-grabs from videos. The facial expression of the animals is sometimes referred to as the pain face.

Ultrasonic vocalizations (USVs) occur at frequencies ranging from approximately 20–100 kHz. They are emitted by animals such as bats and rodents, and have been extensively studied in rats and mice. As opposed to sonic vocalizations, ultrasonic vocalizations cannot be detected by the human ear. USVs serve as social signals, and are categorized according to their frequency. Different categories of USVs are elicited in response to different situations and varying affective states. The behavioural functions of USVs vary as a rat or mouse pup reaches the juvenile/adult stage of their development. The brain mechanisms behind calling behaviour have also been studied, and some studies have used pharmacological manipulation.
















