
Infrared is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with waves that are just longer than those of red light, so IR is invisible to the human eye. IR is generally understood to include wavelengths from around 750 nm (400 THz) to 1 mm (300 GHz). IR is commonly divided between longer-wavelength thermal IR, emitted from terrestrial sources, and shorter-wavelength IR or near-IR, part of the solar spectrum. Longer IR wavelengths (30–100 μm) are sometimes included as part of the terahertz radiation band. Almost all black-body radiation from objects near room temperature is in the IR band. As a form of electromagnetic radiation, IR carries energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon.

Spectroscopy is the field of study that measures and interprets electromagnetic spectrum. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

An optical spectrometer is an instrument used to measure properties of light over a specific portion of the electromagnetic spectrum, typically used in spectroscopic analysis to identify materials. The variable measured is most often the irradiance of the light but could also, for instance, be the polarization state. The independent variable is usually the wavelength of the light or a closely derived physical quantity, such as the corresponding wavenumber or the photon energy, in units of measurement such as centimeters, reciprocal centimeters, or electron volts, respectively.
Atomic, molecular, and optical physics (AMO) is the study of matter–matter and light–matter interactions, at the scale of one or a few atoms and energy scales around several electron volts. The three areas are closely interrelated. AMO theory includes classical, semi-classical and quantum treatments. Typically, the theory and applications of emission, absorption, scattering of electromagnetic radiation (light) from excited atoms and molecules, analysis of spectroscopy, generation of lasers and masers, and the optical properties of matter in general, fall into these categories.
Infrared astronomy is a sub-discipline of astronomy which specializes in the observation and analysis of astronomical objects using infrared (IR) radiation. The wavelength of infrared light ranges from 0.75 to 300 micrometers, and falls in between visible radiation, which ranges from 380 to 750 nanometers, and submillimeter waves.

The W. M. Keck Observatory is an astronomical observatory with two telescopes at an elevation of 4,145 meters (13,600 ft) near the summit of Mauna Kea in the U.S. state of Hawaii. Both telescopes have 10 m (33 ft) aperture primary mirrors, and, when completed in 1993 and 1996, they were the largest optical reflecting telescopes in the world. They have been the third and fourth largest since 2006.
Astronomical spectroscopy is the study of astronomy using the techniques of spectroscopy to measure the spectrum of electromagnetic radiation, including visible light, ultraviolet, X-ray, infrared and radio waves that radiate from stars and other celestial objects. A stellar spectrum can reveal many properties of stars, such as their chemical composition, temperature, density, mass, distance and luminosity. Spectroscopy can show the velocity of motion towards or away from the observer by measuring the Doppler shift. Spectroscopy is also used to study the physical properties of many other types of celestial objects such as planets, nebulae, galaxies, and active galactic nuclei.

Astrophysics is a science that employs the methods and principles of physics and chemistry in the study of astronomical objects and phenomena. As one of the founders of the discipline, James Keeler, said, astrophysics "seeks to ascertain the nature of the heavenly bodies, rather than their positions or motions in space–what they are, rather than where they are", which is studied in celestial mechanics.

Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.

Ira Sprague Bowen was an American physicist and astronomer. In 1927 he discovered that nebulium was not really a chemical element but instead doubly ionized oxygen.
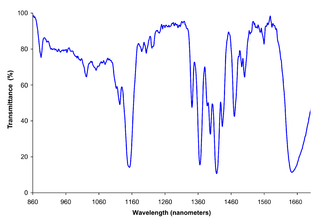
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum. Typical applications include medical and physiological diagnostics and research including blood sugar, pulse oximetry, functional neuroimaging, sports medicine, elite sports training, ergonomics, rehabilitation, neonatal research, brain computer interface, urology, and neurology. There are also applications in other areas as well such as pharmaceutical, food and agrochemical quality control, atmospheric chemistry, combustion research and knowledge.
Curtis Judson Humphreys was an American physicist born in Alliance, Ohio, USA and educated at the University of Michigan. He was chief of the Radiometry Section of the U.S. Navy during the 1940s. He is famous for discovering the Humphreys series of the hydrogen atom.

Heinrich Rubens was a German physicist. He is known for his measurements of the energy of black-body radiation which led Max Planck to the discovery of his radiation law. This was the genesis of quantum theory.
Gerhart "Gerry" Neugebauer was an American astronomer known for his pioneering work in infrared astronomy.
Richard C. Lord (1910–1989) was an American chemist best known for his work in the field of spectroscopy.

Anders Jonas Ångström was a Swedish physicist and one of the founders of the science of spectroscopy.

Modern spectroscopy in the Western world started in the 17th century. New designs in optics, specifically prisms, enabled systematic observations of the solar spectrum. Isaac Newton first applied the word spectrum to describe the rainbow of colors that combine to form white light. During the early 1800s, Joseph von Fraunhofer conducted experiments with dispersive spectrometers that enabled spectroscopy to become a more precise and quantitative scientific technique. Since then, spectroscopy has played and continues to play a significant role in chemistry, physics and astronomy. Fraunhofer observed and measured dark lines in the Sun's spectrum, which now bear his name although several of them were observed earlier by Wollaston.
Dale P. Cruikshank is an astronomer and planetary scientist in the Astrophysics Branch at NASA Ames Research Center. His research specialties are spectroscopy and radiometry of planets and small bodies in the Solar System. These small bodies include comets, asteroids, planetary satellites, dwarf planets, and objects in the region beyond Neptune. He uses spectroscopic observations made with ground-based and space-based telescopes, as well as interplanetary spacecraft, to identify and study the ices, minerals, and organic materials that compose the surfaces of planets and small bodies.
Guido Münch Paniagua was a Mexican astronomer and astrophysicist.
R. Robert Brattain was an American physicist at Shell Development Company. He was involved in a number of secret projects during World War II. He is recognized as one of America's leading infrared spectroscopists for his work in designing several models of spectrophotometer, and for using the infrared spectrophotometer to determine the β-lactam structure of penicillin. His instrumentation work was essential to the subsequent study and understanding of structures in organic chemistry.















