Related Research Articles

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.

Vegetable oils, or vegetable fats, are oils extracted from seeds or from other parts of fruits. Like animal fats, vegetable fats are mixtures of triglycerides. Soybean oil, grape seed oil, and cocoa butter are examples of seed oils, or fats from seeds. Olive oil, palm oil, and rice bran oil are examples of fats from other parts of fruits. In common usage, vegetable oil may refer exclusively to vegetable fats which are liquid at room temperature. Vegetable oils are usually edible.

Coconut oil is an edible oil derived from the kernels, meat, and milk of the coconut palm fruit. Coconut oil is a white solid fat below around 25 °C (77 °F), and a clear thin liquid oil in warmer climates. Unrefined varieties have a distinct coconut aroma. Coconut oil is used as a food oil, and in industrial applications for cosmetics and detergent production. The oil is rich in medium-chain fatty acids.

Hemp oil is oil obtained by pressing hemp seeds. Cold pressed, unrefined hemp oil is dark to clear light green in color, with a nutty flavor. The darker the color, the grassier the flavour. It should not be confused with hash oil, a tetrahydrocannabinol-containing oil made from the Cannabis flower.
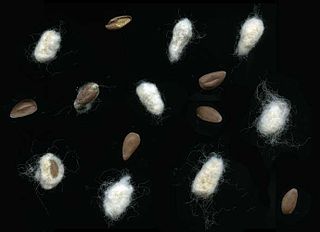
Cottonseed oil is cooking oil from the seeds of cotton plants of various species, mainly Gossypium hirsutum and Gossypium herbaceum, that are grown for cotton fiber, animal feed, and oil.
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This involves vegetable or animal fats and oils being reacted with short-chain alcohols. The alcohols used should be of low molecular weight. Ethanol is the most used because of its low cost, however, greater conversions into biodiesel can be reached using methanol. Although the transesterification reaction can be catalyzed by either acids or bases, the base-catalyzed reaction is more common. This path has lower reaction times and catalyst cost than those acid catalysis. However, alkaline catalysis has the disadvantage of high sensitivity to both water and free fatty acids present in the oils. August 10 is international biodiesel day
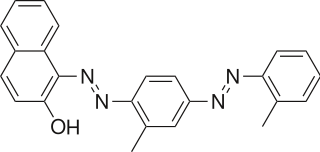
Sudan IV (C24H20N4O) is a lysochrome (fat-soluble dye) diazo dye used for the staining of lipids, triglycerides and lipoproteins on frozen paraffin sections. It has the appearance of reddish brown crystals with melting point 199 °C and maximum absorption at 520(357) nm.
In chemistry, acid value is a number used to quantify the acidity of a given chemical substance. It is the quantity of base, expressed as milligrams of KOH required to neutralize the acidic constituents in 1 gram of a sample. The acid value measures the acidity of water-insoluble substances like oils, fats, waxes and resins, which do not have a pH value.

Rice bran oil is the oil extracted from the hard outer brown layer of rice called bran. It is known for its high smoke point of 232 °C (450 °F) and mild flavor, making it suitable for high-temperature cooking methods such as stir frying and deep frying. It is popular as a cooking oil in East Asia, the Indian subcontinent, and Southeast Asia including India, Nepal, Bangladesh, Indonesia, Japan, Southern China and Malaysia.
Supercritical fluid extraction (SFE) is the process of separating one component (the extractant) from another (the matrix) using supercritical fluids as the extracting solvent. Extraction is usually from a solid matrix, but can also be from liquids. SFE can be used as a sample preparation step for analytical purposes, or on a larger scale to either strip unwanted material from a product (e.g. decaffeination) or collect a desired product (e.g. essential oils). These essential oils can include limonene and other straight solvents. Carbon dioxide (CO2) is the most used supercritical fluid, sometimes modified by co-solvents such as ethanol or methanol. Extraction conditions for supercritical carbon dioxide are above the critical temperature of 31 °C and critical pressure of 74 bar. Addition of modifiers may slightly alter this. The discussion below will mainly refer to extraction with CO2, except where specified.

Corn oil or maize oil (British) is oil extracted from the germ of corn (maize). Its main use is in cooking, where its high smoke point makes refined corn oil a valuable frying oil. It is also a key ingredient in some margarines. Corn oil is generally less expensive than most other types of vegetable oils.

Sunflower oil is the non-volatile oil pressed from the seeds of the sunflower. Sunflower oil is commonly used in food as a frying oil, and in cosmetic formulations as an emollient.
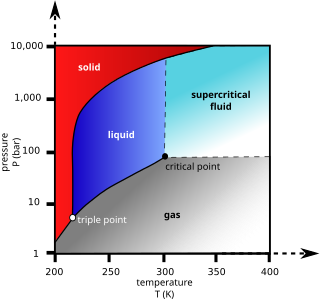
Supercritical carbon dioxide is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic and lipophilic. Oils are usually flammable and surface active. Most oils are unsaturated lipids that are liquid at room temperature.

Fragrance extraction refers to the separation process of aromatic compounds from raw materials, using methods such as distillation, solvent extraction, expression, sieving, or enfleurage. The results of the extracts are either essential oils, absolutes, concretes, or butters, depending on the amount of waxes in the extracted product.
In the food industry and biochemistry, interesterification (IE) is a process that rearranges the fatty acids of a fat product, typically a mixture of triglycerides. The process implies breaking and reforming the ester bonds C–O–C that connect the fatty acid chains to the glycerol hubs of the fat molecules. The reactions involve catalysts, either inorganic chemicals or enzymes.
Single cell oil, also known as Microbial oil consists of the intracellular storage lipids, triacyglycerols. It is similar to vegetable oil, another biologically produced oil. They are produced by oleaginous microorganisms, which is the term for those bacteria, molds, algae and yeast, which can accumulate 20% to 80% lipids of their biomass. The accumulation of lipids take place by the end of logarithmic phase and continues during station phase until carbon source begins to reduce with nutrition limitation.

Cooking oil is a plant or animal liquid fat used in frying, baking, and other types of cooking. Oil allows higher cooking temperatures than water, making cooking faster and more flavorful, while likewise distributing heat, reducing burning and uneven cooking. It sometimes imparts its own flavor. Cooking oil is also used in food preparation and flavoring not involving heat, such as salad dressings and bread dips.
Edible oil refining is a set of processes or treatments necessary to turn vegetable raw oil into edible oil.
References
- ↑ Chalapud, Mayra C.; Baümler, Erica R.; Carelli, Amalia A. (February 2017). "Characterization of waxes and residual oil recovered from sunflower oil winterization waste". European Journal of Lipid Science and Technology. 119 (2): 1500608. doi:10.1002/ejlt.201500608.
- ↑ Abe, Masahiro; Komatsu, Hiroyuki; Yamagiwa, Kazuaki; Tajima, Hideo (February 2018). "Evaluation of the separation of saturated fatty acid methyl esters obtained from additive winterization using a nonionic surfactant". Fuel. 214: 607–613. doi:10.1016/j.fuel.2017.11.066.
- ↑ Vijayan, Sandhya K.; Naveena Victor, Mary; Sudharsanam, Abinandan; et al. (March 2018). "Winterization studies of different vegetable oil biodiesel". Bioresource Technology Reports. 1: 50–55. doi:10.1016/j.biteb.2018.02.005.
- ↑ "The Differences Between Winterized and Distillate Cannabis Oil". BAS Research - The Oil In Cannabis Brands™. 2019-03-26. Retrieved 2019-04-09.
- ↑ Ribeiro Grijó, Daniel; Vieitez Osorio, Ignacio Alberto; Cardozo-Filho, Lúcio (March 2019). "Supercritical Extraction Strategies Using CO2 and Ethanol to Obtain Cannabinoid Compounds from Cannabis Hybrid Flowers". Journal of CO2 Utilization. 30: 241–248. doi: 10.1016/j.jcou.2018.12.014 .