Related Research Articles

Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy.

An androgen is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This includes the embryological development of the primary male sex organs, and the development of male secondary sex characteristics at puberty. Androgens are synthesized in the testes, the ovaries, and the adrenal glands.

Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effects are mediated by slow genomic mechanisms through nuclear receptors as well as by fast nongenomic mechanisms through membrane-associated receptors and signaling cascades. The polypeptide hormones luteinizing hormone, follicle-stimulating hormone and gonadotropin-releasing hormone are usually not regarded as sex hormones, although they play major sex-related roles.

Estrogen receptors (ERs) are a group of proteins found inside cells. They are receptors that are activated by the hormone estrogen (17β-estradiol). Two classes of ER exist: nuclear estrogen receptors, which are members of the nuclear receptor family of intracellular receptors, and membrane estrogen receptors (mERs), which are mostly G protein-coupled receptors. This article refers to the former (ER).

The hypothalamic–pituitary–gonadal axis refers to the hypothalamus, pituitary gland, and gonadal glands as if these individual endocrine glands were a single entity. Because these glands often act in concert, physiologists and endocrinologists find it convenient and descriptive to speak of them as a single system.

Homosalate is an organic compound used in some sunscreens. It is made by the Fischer–Speier esterification of salicylic acid and 3,3,5-trimethylcyclohexanol, the latter being a hydrogenated derivative of isophorone. Contained in 45% of U.S. sunscreens, it is used as a chemical UV filter. The salicylic acid portion of the molecule absorbs ultraviolet rays with a wavelength from 295 nm to 315 nm, protecting the skin from sun damage. The hydrophobic trimethyl cyclohexyl group provides greasiness that prevents it from dissolving in water.

Trenbolone acetate, sold under brand names such as Finajet and Finaplix among others, is an androgen and anabolic steroid (AAS) medication which is used in veterinary medicine, specifically to increase the profitability of livestock by promoting muscle growth in cattle. It is given by injection into muscle.
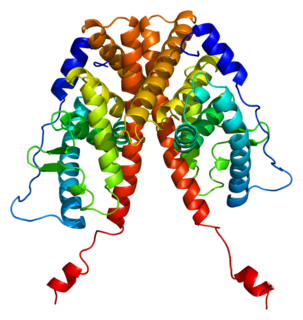
Estrogen insensitivity syndrome (EIS), or estrogen resistance, is a form of congenital estrogen deficiency or hypoestrogenism which is caused by a defective estrogen receptor (ER) – specifically, the estrogen receptor alpha (ERα) – that results in an inability of estrogen to mediate its biological effects in the body. Congenital estrogen deficiency can alternatively be caused by a defect in aromatase, the enzyme responsible for the biosynthesis of estrogens, a condition which is referred to as aromatase deficiency and is similar in symptomatology to EIS.
The estrogen receptor test (ERT) uses the estrogen receptor (ER) tumor marker that allows for immunohistochemical techniques to be performed for diagnostic purposes. Immunohistochemistry (IHC) techniques involve the selective identification of antigen proteins by exploiting these antigen-antibody relationships to characterize your analyte of interest. Previously, the ligand binding assay has been used in the determination of ER activity, however this method was limited because of the requirement of large quantities of fresh tissue needed for each assay. IHC serves as a more efficient methods as this technique allows for the morphology of the tissue to be observed in a tumor-specific manner. This increases the practicability of this technique as in many cases, patients’ tissue samples are limited in the applications of biomarker analysis. Anti-estrogen receptor antibodies were among the first of biomarkers which introduced a semi-quantitative assessment of the ER activity. Today, ER analysis is one of many routinely performed immunohistochemical assays performed to classify the hormone receptor status and to serve as a means of insight in the determination of cancer prognosis and management.
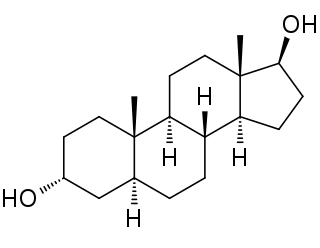
3α-Androstanediol also known as 5α-androstane-3α,17β-diol and sometimes shortened in the literature to 3α-diol, is an endogenous steroid hormone and neurosteroid and a metabolite of androgens like dihydrotestosterone (DHT).

3β-Androstanediol, also known as 5α-androstane-3β,17β-diol, and sometimes shortened in the literature to 3β-diol, is an endogenous steroid hormone and a metabolite of androgens like dehydroepiandrosterone (DHEA) and dihydrotestosterone (DHT).
Toxicodynamics, termed pharmacodynamics in pharmacology, describes the dynamic interactions of a toxicant with a biological target and its biological effects. A biological target, also known as the site of action, can be binding proteins, ion channels, DNA, or a variety of other receptors. When a toxicant enters an organism, it can interact with these receptors and produce structural or functional alterations. The mechanism of action of the toxicant, as determined by a toxicant’s chemical properties, will determine what receptors are targeted and the overall toxic effect at the cellular level and organismal level.
Chemical Activated LUciferase gene eXpression (CALUX) is a ligand-dependent nuclear receptor-based bioassay used in the detection of specific chemicals or classes of chemicals in samples. It consists of a modified cell line that has been stably transfected with a DNA construct with a luciferase reporter gene under control of receptor-specific DNA response elements that can stimulate transcription of the inserted luciferase gene and produce the light-generating enzyme which can be easily measured. The DNA response elements can be varied in order to provide binding sites for other receptors that are regulated by a chemical or class of chemicals of interest that want to be detected. Thus, numerous CALUX bioassays have been developed for detection of diverse chemicals of interest. Most applications have been directed toward the detection of environmentally harmful chemicals, such as those affecting the endocrine system.
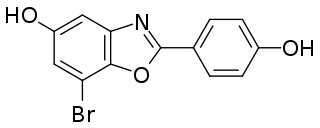
WAY-200070 is a synthetic, nonsteroidal, highly selective agonist of ERβ. It possesses 68-fold selectivity for ERβ over ERα (EC50 = 2 nM and 155 nM, respectively). WAY-200070 has been found to enhance serotonergic and dopaminergic neurotransmission in the central nervous system, and produces antidepressant- and anxiolytic-like effects in animals. It has been proposed as a potential novel antidepressant/anxiolytic agent. WAY-200070 has also been found to produce antidiabetic effects in animals, and may also be beneficial for the treatment of certain inflammatory conditions.

1-Keto-1,2,3,4-tetrahydrophenanthrene (THP-1), or 1,2,3,4-tetrahydrophenanthren-1-one, is a synthetic steroid-like compound which was reported to be the first synthetic estrogen, or the first synthetic compound identified with estrogenic activity. It was first synthesized in 1933 by Cook et al. and was tested due to its similarity to the presumed chemical structure of estrone. Upon reassessment many decades later, the compound was found to bind only weakly to the estrogen receptors, and, unexpectedly, did not actually have functional activity as an estrogen or antiestrogen in vitro or in vivo. It did, however, show some androgenic and antiandrogenic activity in vitro.
E-SCREEN is a cell proliferation assay based on the enhanced proliferation of human breast cancer cells (MCF-7) in the presence of estrogen active substances. The E-SCREEN test is a tool to easily and rapidly assess estrogenic activity of suspected xenoestrogens. This bioassay measures estrogen-induced increase of the number of human breast cancer cell, which is biologically equivalent to the increase of mitotic activity in tissues of the genital tract. It was originally developed by Soto et al and was included in the first version of the OECD Conceptual Framework for Testing and Assessment of Endocrine Disrupters published in 2012. However, due to failed validation, it was not included in the updated version of the framework published in 2018.
A hormone-sensitive cancer, or hormone-dependent cancer, is a type of cancer that is dependent on a hormone for growth and/or survival. Examples include breast cancer, which is dependent on estrogens like estradiol, and prostate cancer, which is dependent on androgens like testosterone.

Estradiol 3-glucuronide 17β-sulfate (E2-3G-17S) is an endogenous estrogen conjugate and metabolite of estradiol. It is related to estradiol 3-sulfate and estradiol 17β-glucuronide. Estradiol 3-glucuronide 17β-sulfate has 0.0001% of the relative binding affinity of estradiol for the ERα, one of the two estrogen receptors (ERs). It shows less than one million-fold lower potency in activating the estrogen receptors relative to estradiol in vitro.

11β-Chloromethylestradiol is a synthetic steroidal estrogen which was never marketed. It has very high affinity for the estrogen receptor and dissociates from it relatively slowly. It was originally thought that 11β-CME2 might be a covalent ligand of the estrogen receptors, but its binding was subsequently shown to be fully reversible. The relative binding affinity of 11β-CME2 for the estrogen receptors ranges from 230 to 3,320% of that of estradiol depending on the study. 11β-CME2 also has about 14% of the relative binding affinity of estradiol for sex hormone-binding globulin (SHBG). The compound has been developed as a radiolabel for the ERs.
References
- 1 2 Routledge, E.J. and Sumpter, J.P. (1996). Environ. Toxicol. Chem. 15(3): 241-248.
- 1 2 Sohoni, P. and Sumpter, J.P. (1998). Endocrinol. 158, 327-339
- ↑ Purvis, I. J., Chotai, D., Dykes, C. W., Lubahn, D. B., French, F. S., Wilson, E. M., and Hobden, A. N. (1991). Gene 106,35–42.
- 1 2 3 Schultis T. and Metzger J.W., 2004. Chemosphere 57, 1649-1655
- ↑ Xenometrix AG, Allschwil, Switzerland.
- ↑ NIH Publication No: 03-4503: Test Method Evaluation Report: Review of In Vitro Endocrine Disruptor Screening Assays (2003):https://ntp.niehs.nih.gov/iccvam/docs/endo_docs/edfinalrpt0503/edfinrpt.pdf
- ↑ Kolle, S.N., Kamp H.G., Huener H.A., Knickel J., Verlohner A., Woitkowiak C., Landsiedel R., van Ravenzwaay B. (2010). Toxicol. In vitro 24(7): 2030-2040