In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (—OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.

An oxime is a chemical compound belonging to the imines, with the general formula RR'C=NOH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an α,β-unsaturated carbonyl compound containing an electron withdrawing group. It belongs to the larger class of conjugate additions and is widely used for the mild formation of C–C bonds. Many asymmetric variants exist.
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds.

Aleksandr Pavlovich Dianin was a Russian chemist from Saint Petersburg. He carried out studies on phenols and discovered a phenol derivative now known as bisphenol A and the accordingly named Dianin's compound. He was married to the adopted daughter of fellow chemist Alexander Borodin. In 1887, Dianin succeeded his father-in-law as chair of the Chemistry Department at the Imperial Medical-Surgical Academy in St. Petersburg.

The Reimer–Tiemann reaction is a chemical reaction used for the ortho-formylation of phenols; with the simplest example being the conversion of phenol to salicylaldehyde. The reaction was discovered by Karl Reimer and Ferdinand Tiemann. The Reimer in question was Karl Reimer (1845-1883) not the lesser known Carl Ludwig Reimer (1856-1921).

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.
The Stollé synthesis is a series of chemical reactions that produce oxindoles from anilines and α-haloacid chlorides.
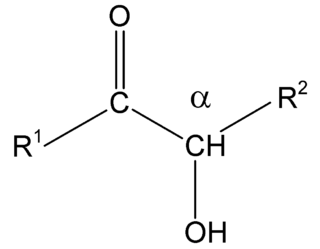
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups.
The Elbs persulfate oxidation is the organic reaction of phenols with alkaline potassium persulfate to form para-diphenols.
The Schiemann reaction is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to fluorobenzene and some related derivatives, including 4-fluorobenzoic acid.
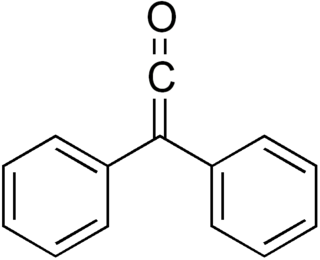
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumule. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.
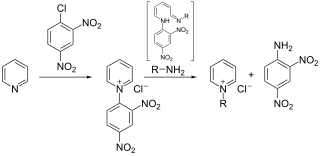
The Zincke reaction is an organic reaction in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine, named after Theodor Zincke.

The Wolffenstein–Böters reaction is an organic reaction converting benzene to picric acid by a mixture of aqueous nitric acid and mercury(II) nitrate.
The Wohl–Aue reaction is an organic reaction between an aromatic nitro compound and an aniline to form a phenazine in presence of an alkali base. An example is the reaction between nitrobenzene and aniline:

Zincke aldehydes, or 5-aminopenta-2,4-dienals, are the product of the reaction of a pyridinium salt with two equivalents of any secondary amine, followed by basic hydrolysis. Using secondary amines the Zincke reaction takes on a different shape forming Zincke aldehydes in which the pyridine ring is ring-opened with the terminal iminium group hydrolyzed to an aldehyde. The use of the dinitrophenyl group for pyridine activation was first reported by Theodor Zincke. The use of cyanogen bromide for pyridine activation was independently reported by W. König:

Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole.

Durene, or 1,2,4,5-tetramethylbenzene, is an organic compound with the formula C6H2(CH3)4. It is a colourless solid with a sweet odor. The compound is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and isodurene (1,2,3,5-tetramethylbenzene). Durene has an unusually high melting point (79.2 °C), reflecting its high molecular symmetry.
The Buchner ring expansion is a two-step organic C-C bond forming reaction used to access 7-membered rings. The first step involves formation of a carbene from ethyl diazoacetate, which cyclopropanates an aromatic ring. The ring expansion occurs in the second step, with an electrocyclic reaction opening the cyclopropane ring to form the 7-membered ring.
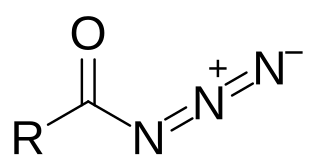
Acyl azides are carboxylic acid derivatives with the general formula RCON3. These compounds, which are a subclass of organic azides, are generally colorless.