
Hydrazones are a class of organic compounds with the structure R
1R
2C=NNH
2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH
2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.

A dicarbonyl is a molecule containing two carbonyl (C=O) groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.

An imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
Enols, or more formally, alkenols, are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.

Acetylacetone is an organic compound with the chemical formula CH3COCH2COCH3. It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer CH3C(O)CH=(OH)CH3. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion, a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887.
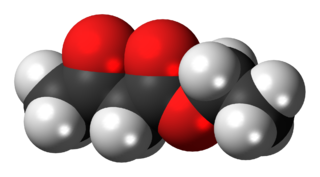
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. It is used as a flavoring for food.

The Biginelli reaction is a multiple-component chemical reaction that creates 3,4-dihydropyrimidin-2(1H)-ones 4 from ethyl acetoacetate 1, an aryl aldehyde, and urea 3. It is named for the Italian chemist Pietro Biginelli.
A nitrone is a functional group in organic chemistry consisting of an N-oxide of an imine. The general structure is R1R2C=NR3+O− where R3 is not H. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal [3+3] cycloadditions to form 6-membered rings, as well as formal [5+2] cycloadditions to form 7-membered rings. Nitrones should not be confused with nitrenes.
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

Etonitazene is an analgesic drug, first reported in 1957, that has been shown to have approximately one thousand to one thousand five hundred times the potency of morphine in animals.

The benzhydryl compounds are a group of organic compounds whose parent structures include diphenylmethane, with any number of attached substituents, including bridges. This group typically excludes compounds in which either benzene is fused to another ring or includes a heteroatom, or where the methane connects to three or four benzenes.

Aporphine is an alkaloid with the chemical formula C17H17N. The IUPAC name of aporphine is 6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole.

Sydnones are mesoionic heterocyclic chemical compounds possessing a 1,2,3-oxadiazole core with a keto group in the 5 position. Like other mesoionic compounds they are di-polar, possessing both positive and negative charges which are delocalized across the ring. Recent computational studies have indicated that sydnones and other similar mesoionic compounds are nonaromatic, "though well-stabilized in two separate regions by electron and charge delocalization." Sydnones are heterocyclic compounds named after the city of Sydney, Australia.

Sir Jocelyn Field Thorpe FRS was a British chemist who made major contributions to organic chemistry, including the Thorpe-Ingold effect and three named reactions.

The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould-Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline. The Gould-Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.

Keto acids or ketoacids are organic compounds that contain a carboxylic acid group and a ketone group. In several cases, the keto group is hydrated. The alpha-keto acids are especially important in biology as they are involved in the Krebs citric acid cycle and in glycolysis.

Tetronic acid is a chemical compound, classified as a γ-lactone, with the molecular formula C4H4O3.




















