
Colombia, officially the Republic of Colombia, is a sovereign state largely situated in the northwest of South America, with territories in Central America. Colombia shares a border to the northwest with Panama, to the east with Venezuela and Brazil and to the south with Ecuador and Peru. It shares its maritime limits with Costa Rica, Nicaragua, Honduras, Jamaica, Haiti, and the Dominican Republic. Colombia is a unitary, constitutional republic comprising thirty-two departments, with the capital in Bogota.

Carbon dioxide (chemical formula CO2) is a colorless gas with a density about 60% higher than that of dry air. Carbon dioxide consists of a carbon atom covalently double bonded to two oxygen atoms. It occurs naturally in Earth's atmosphere as a trace gas. The current concentration is about 0.04% (410 ppm) by volume, having risen from pre-industrial levels of 280 ppm. Natural sources include volcanoes, hot springs and geysers, and it is freed from carbonate rocks by dissolution in water and acids. Because carbon dioxide is soluble in water, it occurs naturally in groundwater, rivers and lakes, ice caps, glaciers and seawater. It is present in deposits of petroleum and natural gas. Carbon dioxide is odorless at normally encountered concentrations. However, at high concentrations, it has a sharp and acidic odor.
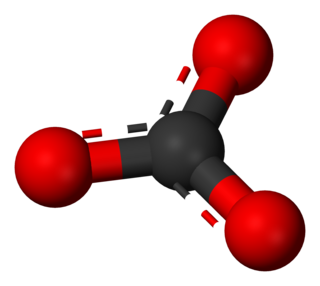
In chemistry, a carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula of CO2−
3. The name may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2.

Carbon monoxide (CO) is a colorless, odorless, and tasteless gas that is slightly less dense than air. It is toxic to animals that use hemoglobin as an oxygen carrier when encountered in concentrations above about 35 ppm, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal biological functions. In the atmosphere, it is spatially variable and short lived, having a role in the formation of ground-level ozone.

The citric acid cycle (CAC) – also known as the TCA cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins, into adenosine triphosphate (ATP) and carbon dioxide. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three segments of the citric acid cycle have been recognized.

Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that can later be released to fuel the organisms' activities. This chemical energy is stored in carbohydrate molecules, such as sugars, which are synthesized from carbon dioxide and water – hence the name photosynthesis, from the Greek φῶς, phōs, "light", and σύνθεσις, synthesis, "putting together". In most cases, oxygen is also released as a waste product. Most plants, most algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies all of the organic compounds and most of the energy necessary for life on Earth.

Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite (most notably as limestone, which is a type of sedimentary rock consisting mainly of calcite) and is the main component of pearls and the shells of marine organisms, snails, and eggs. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale. It is medicinally used as a calcium supplement or as an antacid, but excessive consumption can be hazardous.
Carbonic acid is a chemical compound with the chemical formula H2CO3 (equivalently OC(OH)2). It is also a name sometimes given to solutions of carbon dioxide in water (carbonated water), because such solutions contain small amounts of H2CO3. In physiology, carbonic acid is described as volatile acid or respiratory acid, because it is the only acid excreted as a gas by the lungs. It plays an important role in the bicarbonate buffer system to maintain acid–base homeostasis.

Cellular respiration is a set of metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy in the process, as weak so-called "high-energy" bonds are replaced by stronger bonds in the products. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. Cellular respiration is considered an exothermic redox reaction which releases heat. The overall reaction occurs in a series of biochemical steps, most of which are redox reactions themselves. Although cellular respiration is technically a combustion reaction, it clearly does not resemble one when it occurs in a living cell because of the slow release of energy from the series of reactions.

Sodium bicarbonate (IUPAC name: sodium hydrogen carbonate), commonly known as baking soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). Sodium bicarbonate is a white solid that is crystalline, but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs.

Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, water-soluble salts. All forms have a strongly alkaline taste and give moderately alkaline solutions in water. Historically it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.

PepsiCo, Inc. is an American multinational food, snack, and beverage corporation headquartered in Harrison, New York, in the hamlet of Purchase. PepsiCo has interests in the manufacturing, marketing, and distribution of grain-based snack foods, beverages, and other products. PepsiCo was formed in 1965 with the merger of the Pepsi-Cola Company and Frito-Lay, Inc. PepsiCo has since expanded from its namesake product Pepsi to a broader range of food and beverage brands, the largest of which included an acquisition of Tropicana Products in 1998 and the Quaker Oats Company in 2001, which added the Gatorade brand to its portfolio.

Carbon capture and storage (CCS) (or carbon capture and sequestration or carbon control and sequestration) is the process of capturing waste carbon dioxide (CO2) from large point sources, such as biomass or fossil fuel power plants, transporting it to a storage site, and depositing it where it will not enter the atmosphere, normally an underground geological formation. The aim is to prevent the release of large quantities of CO2 into the atmosphere (from fossil fuel use in power generation and other industries). It is a potential means of mitigating the contribution of fossil fuel emissions to global warming and ocean acidification. Although CO2 has been injected into geological formations for several decades for various purposes, including enhanced oil recovery, the long term storage of CO2 is a relatively new concept. The first commercial example was the Weyburn-Midale Carbon Dioxide Project in 2000. Another example is SaskPower's Boundary Dam. 'CCS' can also be used to describe the scrubbing of CO2 from ambient air as a climate engineering technique.

Global warming is a long-term rise in the average temperature of the Earth's climate system, an aspect of climate change shown by temperature measurements and by multiple effects of the warming. The term commonly refers to the mainly human-caused observed warming since pre-industrial times and its projected continuation, though there were also much earlier periods of global warming. In the modern context the terms global warming and climate change are commonly used interchangeably, but climate change includes both global warming and its effects, such as changes to precipitation and impacts that differ by region. Many of the observed warming changes since the 1950s are unprecedented in the instrumental temperature record, and in historical and paleoclimate proxy records of climate change over thousands to millions of years.

Great Britain is an island in the North Atlantic Ocean off the northwest coast of continental Europe. With an area of 209,331 km2 (80,823 sq mi), it is the largest of the British Isles, the largest European island, and the ninth-largest island in the world. In 2011, Great Britain had a population of about 61 million people, making it the world's third-most populous island after Java in Indonesia and Honshu in Japan. The island of Ireland is situated to the west of Great Britain, and together these islands, along with over 1,000 smaller surrounding islands, form the British Isles archipelago.
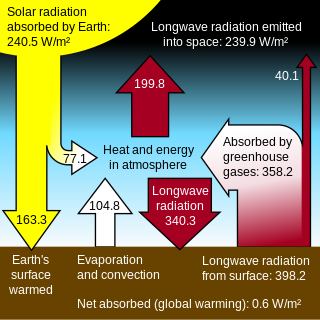
A greenhouse gas is a gas that absorbs and emits radiant energy within the thermal infrared range. Greenhouse gases cause the greenhouse effect. The primary greenhouse gases in Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide and ozone. Without greenhouse gases, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F). The atmospheres of Venus, Mars and Titan also contain greenhouse gases.

Cobalt is a chemical element with symbol Co and atomic number 27. Like nickel, cobalt is found in the Earth's crust only in chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal.

The Organisation for Economic Co-operation and Development is an intergovernmental economic organisation with 36 member countries, founded in 1961 to stimulate economic progress and world trade. It is a forum of countries describing themselves as committed to democracy and the market economy, providing a platform to compare policy experiences, seeking answers to common problems, identify good practices and coordinate domestic and international policies of its members. Most OECD members are high-income economies with a very high Human Development Index (HDI) and are regarded as developed countries. As of 2017, the OECD member states collectively comprised 62.2% of global nominal GDP and 42.8% of global GDP at purchasing power parity. OECD is an official United Nations observer.

















