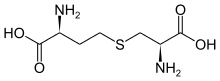
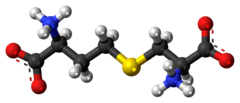
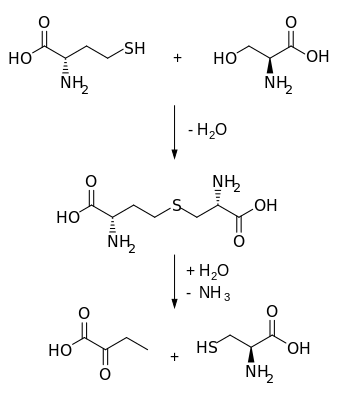
Cysteine is a semiessential proteinogenic amino acid with the formula HOOC−CH(−NH2)−CH2−SH. The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. L‑Cysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems. Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Greek κύστη kýsti, "bladder".

Methionine is an essential amino acid in humans.

Taurine, or 2-aminoethanesulfonic acid, is a non-proteinogenic naturally occurring amino sulfonic acid that is widely distributed in animal tissues. It is a major constituent of bile and can be found in the large intestine, and accounts for up to 0.1% of total human body weight.

Homocysteine or Hcy: is a non-proteinogenic α-amino acid. It is a homologue of the amino acid cysteine, differing by an additional methylene bridge (-CH2-). It is biosynthesized from methionine by the removal of its terminal Cε methyl group. In the body, homocysteine can be recycled into methionine or converted into cysteine with the aid of vitamin B6, B9, and B12.

Homocystinuria (HCU) is an inherited disorder of the metabolism of the amino acid methionine due to a deficiency of cystathionine beta synthase or methionine synthase. It is an inherited autosomal recessive trait, which means a child needs to inherit a copy of the defective gene from both parents to be affected. Symptoms of homocystinuria can also be caused by a deficiency of vitamins B6, B12, or folate.
Cysteine metabolism refers to the biological pathways that consume or create cysteine. The pathways of different amino acids and other metabolites interweave and overlap to creating complex systems.

Sulfur assimilation is the process by which living organisms incorporate sulfur into their biological molecules. In plants, sulfate is absorbed by the roots and then transported to the chloroplasts by the transipration stream where the sulfur are reduced to sulfide with the help of a series of enzymatic reactions. Furthermore, the reduced sulfur is incorporated into cysteine, an amino acid that is a precursor to many other sulfur-containing compounds. In animals, sulfur assimilation occurs primarily through the diet, as animals cannot produce sulfur-containing compounds directly. Sulfur is incorporated into amino acids such as cysteine and methionine, which are used to build proteins and other important molecules.

Cystathionine-β-synthase, also known as CBS, is an enzyme (EC 4.2.1.22) that in humans is encoded by the CBS gene. It catalyzes the first step of the transsulfuration pathway, from homocysteine to cystathionine:

2-Hydroxybutyric acid, is a hydroxybutyric acid with the hydroxyl group on the carbon adjacent to the carboxyl. It is a chiral compound having two enantiomers, D-2-hydroxybutyric acid and L-2-hydroxybutyric acid. Its conjugate base is known as alpha-hydroxybutyrate and α-hydroxybutyrate.

Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structure and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes the deamination of L-serine to yield pyruvate, with the release of ammonia.

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:

Cystathioninuria, also called cystathionase deficiency, is an autosomal recessive metabolic disorder. It is characterized by an abnormal accumulation of plasma cystathionine leading to excess cystathionine in the urine. Hereditary cystathioninuria is associated with the reduced activity of the enzyme cystathionine gamma-lyase. It is considered a biochemical anomaly. This is because it associated with a wide range of diseases and its inconsistency.

The transsulfuration pathway is a metabolic pathway involving the interconversion of cysteine and homocysteine through the intermediate cystathionine. Two transsulfurylation pathways are known: the forward and the reverse.

Cystathionine beta-lyase, also commonly referred to as CBL or β-cystathionase, is an enzyme that primarily catalyzes the following α,β-elimination reaction
The enzyme cysteine lyase catalyzes the chemical reaction

The enzyme methionine γ-lyase (EC 4.4.1.11, MGL) is in the γ-family of PLP-dependent enzymes. It degrades sulfur-containing amino acids to α-keto acids, ammonia, and thiols:

In enzymology, a cystathionine gamma-synthase is an enzyme that catalyzes the formation of cystathionine from cysteine and an activated derivative of homoserine, e.g.:
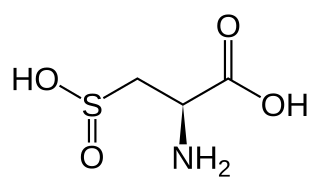
Cysteine sulfinic acid is the organic compound with the nominal formula HO2SCH2CH(NH2)CO2H. It is a rare example of an amino acid bearing a sulfinic acid functional group. It is a white solid that is soluble in water. Like most natural amino acids, it is chiral, only the L-enantiomer occurs in nature, and it exists as the zwitterion at neutral pH. It is an intermediate in cysteine metabolism. It is not a coded amino acid, but is produced post-translationally. Peptides containing the cysteine sulfinic acid residue are substrates for cysteine sulfinic acid reductase.

In molecular biology, the Cys/Met metabolism PLP-dependent enzyme family is a family of proteins including enzymes involved in cysteine and methionine metabolism which use PLP (pyridoxal-5'-phosphate) as a cofactor.

Lanthionine ketimine is a naturally occurring sulfur amino acid metabolite found in the mammalian brain and central nervous system (CNS).