
An endosymbiont or endobiont is an organism that lives within the body or cells of another organism. Typically the two organisms are in a mutualistic relationship. Examples are nitrogen-fixing bacteria, which live in the root nodules of legumes, single-cell algae inside reef-building corals and bacterial endosymbionts that provide essential nutrients to insects.
Nitrogen fixation is a chemical process by which molecular nitrogen (N
2), which has a strong triple covalent bond, is converted into ammonia (NH
3) or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. The nitrogen in air is molecular dinitrogen, a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation or diazotrophy is an important microbe-mediated process that converts dinitrogen (N2) gas to ammonia (NH3) using the nitrogenase protein complex (Nif).

A mycorrhiza is a symbiotic association between a fungus and a plant. The term mycorrhiza refers to the role of the fungus in the plant's rhizosphere, its root system. Mycorrhizae play important roles in plant nutrition, soil biology, and soil chemistry.

Leghemoglobin is an oxygen-carrying phytoglobin found in the nitrogen-fixing root nodules of leguminous plants. It is produced by these plants in response to the roots being colonized by nitrogen-fixing bacteria, termed rhizobia, as part of the symbiotic interaction between plant and bacterium: roots not colonized by Rhizobium do not synthesise leghemoglobin. Leghemoglobin has close chemical and structural similarities to hemoglobin, and, like hemoglobin, is red in colour. It was originally thought that the heme prosthetic group for plant leghemoglobin was provided by the bacterial symbiont within symbiotic root nodules. However, subsequent work shows that the plant host strongly expresses heme biosynthesis genes within nodules, and that activation of those genes correlates with leghemoglobin gene expression in developing nodules.

Rhizobia are diazotrophic bacteria that fix nitrogen after becoming established inside the root nodules of legumes (Fabaceae). To express genes for nitrogen fixation, rhizobia require a plant host; they cannot independently fix nitrogen. In general, they are gram negative, motile, non-sporulating rods.

The Casuarinaceae are a family of dicotyledonous flowering plants placed in the order Fagales, consisting of four genera and 91 species of trees and shrubs native to eastern Africa, Australia, Southeast Asia, Malesia, Papuasia, and the Pacific Islands. At one time, all species were placed in the genus Casuarina. Lawrence Alexander Sidney Johnson separated out many of those species and renamed them into the new genera of Gymnostoma in 1980 and 1982, Allocasuarina in 1982, and Ceuthostoma in 1988, with some additional formal descriptions of new species in each other genus. At the time, it was somewhat controversial. The monophyly of these genera was later supported in a 2003 phylogenetic study of the family. In the Wettstein system, this family was the only one placed in the order Verticillatae. Likewise, in the Engler, Cronquist, and Kubitzki systems, the Casuarinaceae were the only family placed in the order Casuarinales.
Diazotrophs are bacteria and archaea that fix atmospheric nitrogen(N2) in the atmosphere into bioavailable forms such as ammonia.

Ensifer meliloti are an aerobic, Gram-negative, and diazotrophic species of bacteria. S. meliloti are motile and possess a cluster of peritrichous flagella. S. meliloti fix atmospheric nitrogen into ammonia for their legume hosts, such as alfalfa. S. meliloti forms a symbiotic relationship with legumes from the genera Medicago, Melilotus and Trigonella, including the model legume Medicago truncatula. This symbiosis promotes the development of a plant organ, termed a root nodule. Because soil often contains a limited amount of nitrogen for plant use, the symbiotic relationship between S. meliloti and their legume hosts has agricultural applications. These techniques reduce the need for inorganic nitrogenous fertilizers.

Root nodules are found on the roots of plants, primarily legumes, that form a symbiosis with nitrogen-fixing bacteria. Under nitrogen-limiting conditions, capable plants form a symbiotic relationship with a host-specific strain of bacteria known as rhizobia. This process has evolved multiple times within the legumes, as well as in other species found within the Rosid clade. Legume crops include beans, peas, and soybeans.
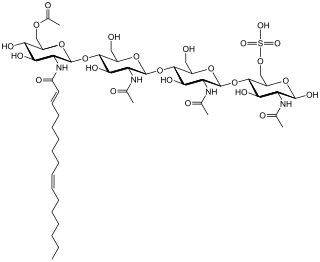
Nod factors, are signaling molecules produced by soil bacteria known as rhizobia in response to flavonoid exudation from plants under nitrogen limited conditions. Nod factors initiate the establishment of a symbiotic relationship between legumes and rhizobia by inducing nodulation. Nod factors produce the differentiation of plant tissue in root hairs into nodules where the bacteria reside and are able to fix nitrogen from the atmosphere for the plant in exchange for photosynthates and the appropriate environment for nitrogen fixation. One of the most important features provided by the plant in this symbiosis is the production of leghemoglobin, which maintains the oxygen concentration low and prevents the inhibition of nitrogenase activity.

Frankia is a genus of nitrogen-fixing bacteria that live in symbiosis with actinorhizal plants, similar to the Rhizobium bacteria found in the root nodules of legumes in the family Fabaceae. Frankia also initiate the forming of root nodules.
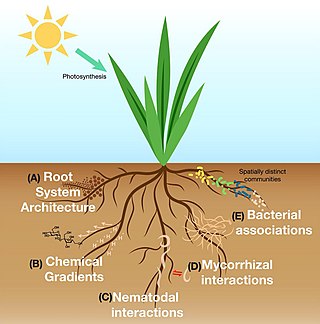
The rhizosphere is the narrow region of soil or substrate that is directly influenced by root secretions and associated soil microorganisms known as the root microbiome. Soil pores in the rhizosphere can contain many bacteria and other microorganisms that feed on sloughed-off plant cells, termed rhizodeposition, and the proteins and sugars released by roots, termed root exudates. This symbiosis leads to more complex interactions, influencing plant growth and competition for resources. Much of the nutrient cycling and disease suppression by antibiotics required by plants occurs immediately adjacent to roots due to root exudates and metabolic products of symbiotic and pathogenic communities of microorganisms. The rhizosphere also provides space to produce allelochemicals to control neighbours and relatives.
Symbiotic bacteria are bacteria living in symbiosis with another organism or each other. For example, rhizobia living in root nodules of legumes provide nitrogen fixing activity for these plants.

Chamaebatia, also known as mountain misery, is a genus of two species of aromatic evergreen shrubs endemic to California. Its English common name derives from early settlers' experience with the plant's dense tangle and sticky, strong-smelling resin. They are actinorhizal, non-legumes capable of nitrogen fixation through symbiosis with the actinobacterium, Frankia.

Bradyrhizobium is a genus of Gram-negative soil bacteria, many of which fix nitrogen. Nitrogen fixation is an important part of the nitrogen cycle. Plants cannot use atmospheric nitrogen (N2); they must use nitrogen compounds such as nitrates.

Casuarina glauca, commonly known as swamp she-oak, swamp buloke, swamp she-oak, marsh sheoak, grey she-oak, grey she-oak, native pine, or guman by the Gadigal people, is a species of flowering plant that is endemic to eastern Australia. It is a dioecious tree that often forms root suckers and has fissured and scaly bark, spreading or drooping branchlets, the leaves reduced to scales in whorls of 12 to 20, the fruit 9–18 mm (0.35–0.71 in) long containing winged seeds (samaras) 3.5–5.0 mm (0.14–0.20 in) long.

The flavans are benzopyran derivatives that use the 2-phenyl-3,4-dihydro-2H-chromene skeleton. They may be found in plants. These compounds include the flavan-3-ols, flavan-4-ols and flavan-3,4-diols (leucoanthocyanidin).

Frankia alni is a Gram-positive species of actinomycete filamentous bacterium that lives in symbiosis with actinorhizal plants in the genus Alnus. It is a nitrogen-fixing bacterium and forms nodules on the roots of alder trees.
Martin Parniske is a German biologist with a specialisation in genetics, microbiology and biochemistry. He is university professor and head of the Institute of Genetics at the Faculty of Biology of the Ludwig Maximilian University of Munich. Parniske's scientific focus is on the molecular interaction between plants and symbiotic and pathogenic organisms including bacteria, fungi, oomycetes and insects.

A symbiosome is a specialised compartment in a host cell that houses an endosymbiont in a symbiotic relationship.

















