Adenosine triphosphate (ATP) is a nucleotide that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" of intracellular energy transfer.

Cellular respiration is the process by which biological fuels are oxidized in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.

Thermogenin is a mitochondrial carrier protein found in brown adipose tissue (BAT). It is used to generate heat by non-shivering thermogenesis, and makes a quantitatively important contribution to countering heat loss in babies which would otherwise occur due to their high surface area-volume ratio.

The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast. It also refers to the space between the inner and outer nuclear membranes of the nuclear envelope, but is often called the perinuclear space. The IMS of mitochondria plays a crucial role in coordinating a variety of cellular activities, such as regulation of respiration and metabolic functions. Unlike the IMS of the mitochondria, the IMS of the chloroplast does not seem to have any obvious function.
Substrate-level phosphorylation is a metabolism reaction that results in the production of ATP or GTP supported by the energy released from another high-energy bond that leads to phosphorylation of ADP or GDP to ATP or GTP (note that the reaction catalyzed by creatine kinase is not considered as "substrate-level phosphorylation"). This process uses some of the released chemical energy, the Gibbs free energy, to transfer a phosphoryl (PO3) group to ADP or GDP. Occurs in glycolysis and in the citric acid cycle.

The inner mitochondrial membrane (IMM) is the mitochondrial membrane which separates the mitochondrial matrix from the intermembrane space.
The mitochondrial permeability transition pore is a protein that is formed in the inner membrane of the mitochondria under certain pathological conditions such as traumatic brain injury and stroke. Opening allows increase in the permeability of the mitochondrial membranes to molecules of less than 1500 daltons in molecular weight. Induction of the permeability transition pore, mitochondrial membrane permeability transition, can lead to mitochondrial swelling and cell death through apoptosis or necrosis depending on the particular biological setting.

Bongkrek acid is a respiratory toxin produced in fermented coconut or corn contaminated by the bacterium Burkholderia gladioli pathovar cocovenenans. It is a highly toxic, heat-stable, colorless, odorless, and highly unsaturated tricarboxylic acid that inhibits the ADP/ATP translocase, also called the mitochondrial ADP/ATP carrier, preventing ATP from leaving the mitochondria to provide metabolic energy to the rest of the cell. Bongkrek acid, when consumed through contaminated foods, mainly targets the liver, brain, and kidneys along with symptoms that include vomiting, diarrhea, urinary retention, abdominal pain, and excessive sweating. Most of the outbreaks are found in Indonesia and China where fermented coconut and corn-based foods are consumed.

The TIM/TOM complex is a protein complex in cellular biochemistry which translocates proteins produced from nuclear DNA through the mitochondrial membrane for use in oxidative phosphorylation. In enzymology, the complex is described as an mitochondrial protein-transporting ATPase, or more systematically ATP phosphohydrolase , as the TIM part requires ATP hydrolysis to work.

Mitochondrial membrane transport proteins, also known as mitochondrial carrier proteins, are proteins which exist in the membranes of mitochondria. They serve to transport molecules and other factors, such as ions, into or out of the organelles. Mitochondria contain both an inner and outer membrane, separated by the inter-membrane space, or inner boundary membrane. The outer membrane is porous, whereas the inner membrane restricts the movement of all molecules. The two membranes also vary in membrane potential and pH. These factors play a role in the function of mitochondrial membrane transport proteins. There are 53 discovered human mitochondrial membrane transporters, with many others that are known to still need discovered.
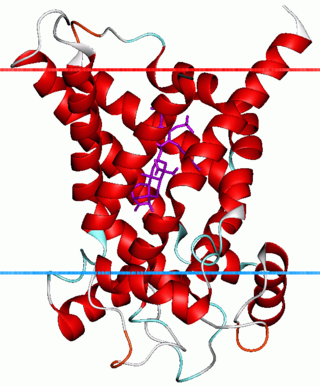
Mitochondrial carriers are proteins from solute carrier family 25 which transfer molecules across the membranes of the mitochondria. Mitochondrial carriers are also classified in the Transporter Classification Database. The Mitochondrial Carrier (MC) Superfamily has been expanded to include both the original Mitochondrial Carrier (MC) family and the Mitochondrial Inner/Outer Membrane Fusion (MMF) family.
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.

An uncoupling protein (UCP) is a mitochondrial inner membrane protein that is a regulated proton channel or transporter. An uncoupling protein is thus capable of dissipating the proton gradient generated by NADH-powered pumping of protons from the mitochondrial matrix to the mitochondrial intermembrane space. The energy lost in dissipating the proton gradient via UCPs is not used to do biochemical work. Instead, heat is generated. This is what links UCP to thermogenesis. However, not every type of UCPs are related to thermogenesis. Although UCP2 and UCP3 are closely related to UCP1, UCP2 and UCP3 do not affect thermoregulatory abilities of vertebrates. UCPs are positioned in the same membrane as the ATP synthase, which is also a proton channel. The two proteins thus work in parallel with one generating heat and the other generating ATP from ADP and inorganic phosphate, the last step in oxidative phosphorylation. Mitochondria respiration is coupled to ATP synthesis, but is regulated by UCPs. UCPs belong to the mitochondrial carrier (SLC25) family.

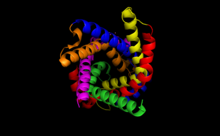
ADP/ATP translocase 1, or adenine nucleotide translocator 1 (ANT1), is an enzyme that in humans is encoded by the SLC25A4 gene.

Tricarboxylate transport protein, mitochondrial, also known as tricarboxylate carrier protein and citrate transport protein (CTP), is a protein that in humans is encoded by the SLC25A1 gene. SLC25A1 belongs to the mitochondrial carrier gene family SLC25. High levels of the tricarboxylate transport protein are found in the liver, pancreas and kidney. Lower or no levels are present in the brain, heart, skeletal muscle, placenta and lung.

ADP/ATP translocase 4 (ANT4) is an enzyme that in humans is encoded by the SLC25A31 gene on chromosome 4. This enzyme inhibits apoptosis by catalyzing ADP/ATP exchange across the mitochondrial membranes and regulating membrane potential. In particular, ANT4 is essential to spermatogenesis, as it imports ATP into sperm mitochondria to support their development and survival. Outside this role, the SLC25AC31 gene has not been implicated in any human disease.

ADP/ATP translocase 3, also known as solute carrier family 25 member 6, is a protein that in humans is encoded by the SLC25A6 gene.

ADP/ATP translocase 2 is a protein that in humans is encoded by the SLC25A5 gene on the X chromosome.
The ATP:ADP Antiporter (AAA) Family is a member of the major facilitator superfamily. Members of the AAA family have been sequenced from bacteria and plants.
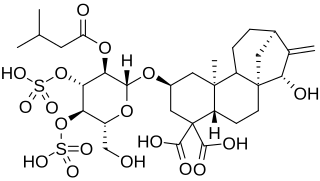
Carboxyatractyloside (CATR) is a highly toxic diterpene glycoside that inhibits the ADP/ATP translocase. It is about 10 times more potent than its analog atractyloside. While atractyloside is effective in the inhibition of oxidative phosphorylation, carboxyatractyloside is considered to be more effective. The effects of carboxyatractyloside on the ADP/ATP translocase are not reversed by increasing the concentration of adenine nucleotides, unlike its counterpart atractyloside. Carboxyatractyloside behavior resembles bongkrekic acid while in the mitochondria. Carboxyatractyloside is poisonous to humans as well as livestock, including cows and horses.