| |
| Clinical data | |
|---|---|
| Other names | Amphenone; 3,3-bis(p-Aminophenyl)butan-2-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H18N2O |
| Molar mass | 254.333 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
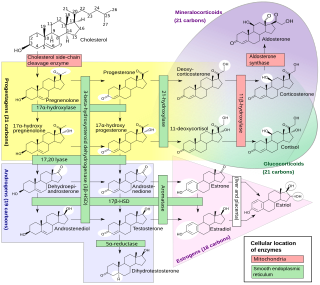
Amphenone B, or simply amphenone, also known as 3,3-bis(p-aminophenyl)butan-2-one, is an inhibitor of steroid hormone and thyroid hormone biosynthesis which was never marketed but has been used as a tool in scientific research to study corticosteroids and the adrenal glands. [1] [2] It acts as competitive inhibitor of 11β-hydroxylase, 17α-hydroxylase, 17,20-lyase, 21-hydroxylase, and 3β-hydroxysteroid dehydrogenase, [1] [2] [3] as well as of cholesterol side-chain cleavage enzyme, [4] [5] thereby inhibiting the production of steroid hormones including glucocorticoids, mineralocorticoids, androgens, and estrogens. [4] [6] In addition, amphenone B inhibits the production of thyroxine by a thiouracil-like mechanism, specifically via inhibition of organic binding of iodine and uptake of iodide by the thyroid gland. [7] [5] [8] [9]
Amphenone B was first synthesized in 1950 and is a diphenylmethane derivative that was derived from the insecticide 2,2-di(p-chlorophenyl)-1,1-dichloroethane (p,p'-DDD), [4] [10] which in 1949 had been found to selectively induce adrenal atrophy. [1] [11] [12] In contrast to p,p'-DDD, which has direct cytotoxic effects on the adrenal glands via an unknown mechanism, [1] amphenone B does not have cytotoxic effects, and instead causes adrenal and thyroid gland hypertrophy due to respective inhibition of corticosteroid and thyroxine biosynthesis, subsequent loss of negative feedback on the hypothalamic-pituitary-adrenal and hypothalamic-pituitary-thyroid axes, and consequent hypersecretion of adrenocorticotropic hormone (ACTH) and thyroid-stimulating hormone (TSH) from the pituitary gland. [1] [2] [4]
Amphenone B has also been found to produce progesterone-like progestogenic effects, including uterine hypertrophy and mammary lobuloalveolar development. [1] [5] [13] [14] These effects occurred even in animals that had been ovariectomized and hypophysectomized, suggesting that amphenone B might be acting directly on the target organs. [1] [5] However, it was found that adrenalectomy abolished the progesterone-like effects of amphenone B on the uterus, whereas those of progesterone were retained in the same experimental conditions, supporting the notion that amphenone B was not actually acting directly on the uterus. [1] Conversely, the progesterone-like effects of amphenone B on the mammary glands were found to persist even in adrenalectomized and ovariectomized animals. [5]
Amphenone B was tested in humans in the mid-1950s as a potential treatment for cortisol-dependent conditions such as Cushing's syndrome and adrenocortical carcinoma. [1] [15] In healthy subjects and patients with adrenocortical carcinoma, the drug was found to be effective in decreasing circulating levels of corticosteroids including cortisol, corticosterone, and aldosterone, [15] as well as in decreasing circulating levels of androgens and estrogens. [1] [6] Moreover, due to reduced aldosterone secretion, it caused marked diuresis and increased urinary sodium excretion. [2] [13] Unfortunately, amphenone B also caused many side effects, some severe, including drowsiness, gastrointestinal disturbances such as heartburn, nausea, and vomiting, morbilliform and pruritic rashes, methemoglobinemia, and hepatotoxicity including impaired liver function and hepatomegaly, [7] and these toxicities, as well as the diversity of its effects on various organs (e.g., also possessing antithyroid and even anesthetic activity), precluded its therapeutic use. [2] [4] [11] [15] [13]
Subsequently, analogues of amphenone B with reduced toxicity and improved specificity were developed. [2] [4] [11] One of the most potent of these was metyrapone (2-methyl-1,2-di(pyridin-3-yl)propan-1-one), [11] a selective inhibitor of 11β-hydroxylase, [2] [6] which was selected for clinical development and was eventually approved and marketed in 1958 as a diagnostic agent for Cushing's syndrome. [1] [4] [16] Another was mitotane (o,p'-DDD, or 1,1-(dichlorodiphenyl)-2,2-dichloroethane), an inhibitor of cholesterol side-chain cleavage enzyme and to a lesser extent of other steroidogenic enzymes, [17] [18] which additionally has selective and direct cytotoxic effects on the adrenal glands similarly to p,p'-DDD, and was introduced in 1960 for the treatment of adrenocortical carcinoma. [4] Aminoglutethimide (3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione), which was originally introduced as an anticonvulsant in 1960, is closely related structurally to amphenone B, [4] [19] and following its introduction, was found to cause adrenal insufficiency in patients due to inhibition of cholesterol side-chain cleavage enzyme and suppression of corticosteroid production. [20] [21] [22] The drug was subsequently repurposed for use in the treatment of metastatic breast cancer and Cushing's syndrome. [20] [22]
Amphenone B was originally thought to be 1,2-bis(p-aminophenyl)-2-methylpropan-1-one, but it was discovered in 1957 that the synthesis of amphenone B was accompanied by an unexpected molecular rearrangement and that the drug was actually 3,3-bis-(p-aminophenyl)butan-2-one. [2] [13] As such, early publications of amphenone B, and some subsequent publications, [5] refer to the drug by the incorrect structure. [2]