Related Research Articles
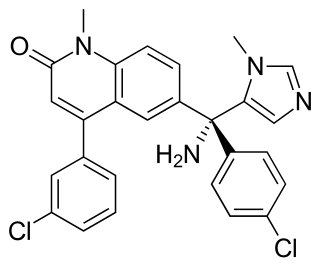
Tipifarnib is a farnesyltransferase inhibitor. Farnesyltransferase inhibitors block the activity of the farnesyltransferase enzyme by inhibiting prenylation of the CAAX tail motif, which ultimately prevents Ras from binding to the membrane, rendering it inactive.

Semaxanib (SU5416) is a tyrosine-kinase inhibitor drug designed by SUGEN as a cancer therapeutic. It is an experimental stage drug, not licensed for use on human patients outside clinical trials. Semaxanib is a potent and selective synthetic inhibitor of the Flk-1/KDR vascular endothelial growth factor (VEGF) receptor tyrosine kinase. It targets the VEGF pathway, and both in vivo and in vitro studies have demonstrated antiangiogenic potential.
Oportuzumab monatox is an antineoplastic. Chemically, oportuzumab it is a single chain variable fragment of a monoclonal antibody which binds to epithelial cell adhesion molecule. The drug is produced by Viventia Bio, a Canadian company with laboratories in Winnipeg, Canada and headquarters in Philadelphia, Pennsylvania, USA. Oportuzumab is fused with Pseudomonas aeruginosa exotoxin A.
Fresolimumab (GC1008) is a human monoclonal antibody and an immunomodulator. It is intended for the treatment of idiopathic pulmonary fibrosis (IPF), focal segmental glomerulosclerosis, and cancer.
Patritumab (INN) is a human monoclonal antibody designed for the treatment of cancer. It acts as an immunomodulator.
Imgatuzumab (INN) is a humanized monoclonal antibody designed for the treatment of cancer. It acts as an immunomodulator.
Lirilumab (INN) is a human monoclonal antibody designed for the treatment of cancer. It binds to KIR2DL1/2L3.
Duligotuzumab (INN) is a humanized IgG1 monoclonal antibody designed for the treatment of cancer. It acts as an immunomodulator and binds to HER3. It is in development by Roche. Clinical development for head and neck cancer and colorectal cancer have been discontinued, but phase I trials in combination with cobimetinib are evaluating safety for treatment of solid tumors.
Ontuxizumab is a humanized rabbit monoclonal antibody designed for the treatment of cancer. It binds to endosialin.
Emibetuzumab (INN) (LY2875358) is a humanized monoclonal antibody designed for the treatment of cancer. It is in phase II trials for patients with NSCLC
Varlilumab is a monoclonal antibody designed for immunotherapy for solid tumors and hematologic malignancies. It is an anti-CD27 antibody and helps activate T-cells.
Lifastuzumab vedotin is a monoclonal antibody designed for the treatment of cancer.
Sofituzumab vedotin is a monoclonal antibody designed for the treatment of ovarian cancer.
Enfortumab vedotin, sold under the brand name Padcev, is an antibody-drug conjugate designed for the treatment of cancer expressing Nectin-4. Enfortumab refers to the monoclonal antibody part, and vedotin refers to the payload drug (MMAE) and the linker.
Seribantumab is a monoclonal antibody designed for the treatment of cancer.
Depatuxizumab mafodotin is an antibody-drug conjugate designed for the treatment of cancer. It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.
Rovalpituzumab tesirine (Rova-T) is an experimental antibody-drug conjugate targeting the protein DLL3 on tumor cells. It was originally developed by Stemcentrx and was purchased by AbbVie. It was tested for use in small-cell lung cancer, but development was terminated after unsuccessful phase III trial.
Carotuximab (INN) (TRC-105) is a chimeric monoclonal antibody designed for the treatment of cancer.
Dostarlimab (INN) is a monoclonal antibody designed for the treatment of cancer.
References
| This monoclonal antibody–related article is a stub. You can help Wikipedia by expanding it. |