
Cotransporters are a subcategory of membrane transport proteins (transporters) that couple the favorable movement of one molecule with its concentration gradient and unfavorable movement of another molecule against its concentration gradient. They enable cotransport and include antiporters and symporters. In general, cotransporters consist of two out of the three classes of integral membrane proteins known as transporters that move molecules and ions across biomembranes. Uniporters are also transporters but move only one type of molecule down its concentration gradient and are not classified as cotransporters.

Glucose transporters are a wide group of membrane proteins that facilitate the transport of glucose across the plasma membrane, a process known as facilitated diffusion. Because glucose is a vital source of energy for all life, these transporters are present in all phyla. The GLUT or SLC2A family are a protein family that is found in most mammalian cells. 14 GLUTS are encoded by human genome. GLUT is a type of uniporter transporter protein.
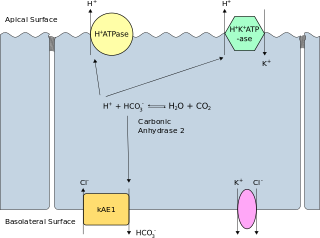
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.
The solute carrier (SLC) group of membrane transport proteins include over 400 members organized into 66 families. Most members of the SLC group are located in the cell membrane. The SLC gene nomenclature system was originally proposed by the HUGO Gene Nomenclature Committee (HGNC) and is the basis for the official HGNC names of the genes that encode these transporters. A more general transmembrane transporter classification can be found in TCDB database.
The Na-K-Cl cotransporter (NKCC) is a protein that aids in the secondary active transport of sodium, potassium, and chloride into cells. In humans there are two isoforms of this membrane transport protein, NKCC1 and NKCC2, encoded by two different genes. Two isoforms of the NKCC1/Slc12a2 gene result from keeping or skipping exon 21 in the final gene product.
In molecular biology, the electroneutral cation-Cl family of proteins are a family of solute carrier proteins. This family includes the products of the Human genes: SLC12A1, SLC12A1, SLC12A2, SLC12A3, SLC12A4, SLC12A5, SLC12A6, SLC12A7, SLC12A8 and SLC12A9.
Pendrin is an anion exchange protein that in humans is encoded by the SLC26A4 gene . Pendrin was initially identified as a sodium-independent chloride-iodide exchanger with subsequent studies showing that it also accepts formate and bicarbonate as substrates. Pendrin is similar to the Band 3 transport protein found in red blood cells. Pendrin is the protein which is mutated in Pendred syndrome, which is an autosomal recessive disorder characterized by sensorineural hearing loss, goiter and a partial organification problem detectable by a positive perchlorate test.

The sulfate transporter is a solute carrier family protein that in humans is encoded by the SLC26A2 gene. SLC26A2 is also called the diastrophic dysplasia sulfate transporter (DTDST), and was first described by Hästbacka et al. in 1994. This sulfate (SO42−) transporter also accepts chloride, hydroxyl ions (OH−), and oxalate as substrates. SLC26A2 is expressed at high levels in developing and mature cartilage, as well as being expressed in lung, placenta, colon, kidney, pancreas and testis.

Sodium bicarbonate cotransporter 3 is a protein which in humans is encoded by the SLC4A7 gene.

Electrogenic sodium bicarbonate cotransporter 1 (NBCe1) is a membrane transport protein that in humans is encoded by the 'SLC4A4' gene.

Anion exchange protein 2 (AE2) is a membrane transport protein that in humans is encoded by the SLC4A2 gene. AE2 is functionally similar to the Band 3 Cl−/HCO3− exchange protein.

Chloride anion exchanger, also known as down-regulated in adenoma, is a protein that in humans is encoded by the SLC26A3 gene.

Anion exchange protein 3 is a membrane transport protein that in humans is encoded by the SLC4A3 gene. AE3 is functionally similar to the Band 3 Cl−/HCO3− exchange protein but it is expressed primarily in brain neurons and in the heart. Like AE2 its activity is sensitive to pH. AE3 mutations have been linked to seizures.

Solute carrier family 26 member 6 is a protein that in humans is encoded by the SLC26A6 gene. It is an anion-exchanger expressed in the apical membrane of the kidney proximal tubule, the apical membranes of the duct cells in the pancreas, and the villi of the duodenum.
Electroneutral sodium bicarbonate exchanger 1 is a protein that in humans is encoded by the SLC4A8 gene.

Anion exchange transporter is a protein that in humans is encoded by the SLC26A7 gene.

Electrogenic sodium bicarbonate cotransporter 4 is a protein that in humans is encoded by the SLC4A5 gene.
The cation-chloride cotransporter (CCC) family is part of the APC superfamily of secondary carriers. Members of the CCC family are found in animals, plants, fungi and bacteria. Most characterized CCC family proteins are from higher eukaryotes, but one has been partially characterized from Nicotiana tabacum, and homologous ORFs have been sequenced from Caenorhabditis elegans (worm), Saccharomyces cerevisiae (yeast) and Synechococcus sp.. The latter proteins are of unknown function. These proteins show sequence similarity to members of the APC family. CCC family proteins are usually large, and possess 12 putative transmembrane spanners (TMSs) flanked by large N-terminal and C-terminal hydrophilic domains.
The sulfate permease (SulP) family is a member of the large APC superfamily of secondary carriers. The SulP family is a large and ubiquitous family of proteins derived from archaea, bacteria, fungi, plants and animals. Many organisms including Bacillus subtilis, Synechocystis sp, Saccharomyces cerevisiae, Arabidopsis thaliana and Caenorhabditis elegans possess multiple SulP family paralogues. Many of these proteins are functionally characterized, and most are inorganic anion uptake transporters or anion:anion exchange transporters. Some transport their substrate(s) with high affinities, while others transport it or them with relatively low affinities. Others may catalyze SO2−
4:HCO−
3 exchange, or more generally, anion:anion antiport. For example, the mouse homologue, SLC26A6, can transport sulfate, formate, oxalate, chloride and bicarbonate, exchanging any one of these anions for another. A cyanobacterial homologue can transport nitrate. Some members can function as channels. SLC26A3 and SLC26A6 can function as carriers or channels, depending on the transported anion. In these porters, mutating a glutamate, also involved in transport in the CIC family, created a channel out of the carrier. It also changed the stoichiometry from 2Cl−/HCO−
3 to 1Cl−/HCO−
3.
Divalent anion:Na+ symporters were found in bacteria, archaea, plant chloroplasts and animals.












