Related Research Articles

A prescription drug is a pharmaceutical drug that is only permitted to be dispensed to those with a medical prescription. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practicing medicine without a license and without sufficient education. Different jurisdictions have different definitions of what constitutes a prescription drug.

Colchicine is a medication used to treat gout and Behçet's disease. In gout, it is less preferred to NSAIDs or steroids. Other uses for colchicine include the management of pericarditis and familial Mediterranean fever. Colchicine is taken by mouth.
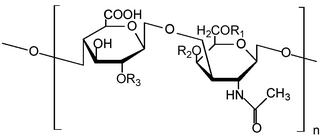
Chondroitin sulfate is a sulfated glycosaminoglycan (GAG) composed of a chain of alternating sugars. It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities. Chondroitin sulfate is an important structural component of cartilage, and provides much of its resistance to compression. Along with glucosamine, chondroitin sulfate has become a widely used dietary supplement for treatment of osteoarthritis, although large clinical trials failed to demonstrate any symptomatic benefit of chondroitin.
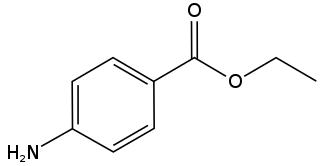
Benzocaine, sold under the brand name Orajel amongst others, is a local anesthetic, belonging to the amino ester drug class, commonly used as a topical painkiller or in cough drops. It is the active ingredient in many over-the-counter anesthetic ointments such as products for oral ulcers. It is combined with antipyrine to form A/B ear drops. In the US, products containing benzocaine for oral application are contraindicated in children younger than two years old. In the European Union, the contraindication applies to children under 12 years of age.

Ofloxacin is a quinolone antibiotic useful for the treatment of a number of bacterial infections. When taken by mouth or injection into a vein, these include pneumonia, cellulitis, urinary tract infections, prostatitis, plague, and certain types of infectious diarrhea. Other uses, along with other medications, include treating multidrug resistant tuberculosis. An eye drop may be used for a superficial bacterial infection of the eye and an ear drop may be used for otitis media when a hole in the ear drum is present.

Phenazone is an analgesic, antipyretic and anti-inflammatory drug. While it predates the term, it is often classified as a nonsteroidal anti-inflammatory drug (NSAID). Phenazone was one of the earliest synthetic medications — when it was patented in 1883, the only synthetic medical chemicals on the market were chloral hydrate, a sedative, trimethylamine, and iodol (tetraiodopyrrol), an early antiseptic. One of the earliest widely used analgesics and antipyretics, phenazone was gradually replaced in common use by other medications including phenacetin, aspirin, paracetamol and modern NSAIDs such as ibuprofen. However, it is still available in several countries either as an over-the-counter or prescribed drug.
Burow's solution is an aqueous solution of aluminium triacetate. It is available in the U.S. as an over-the-counter drug for topical administration, with brand names including Domeboro, Domeboro Otic, Star-Otic, and Borofair. The preparation has astringent and antibacterial properties and may be used to treat a number of skin conditions, including insect bites and stings, rashes caused by poison ivy and poison sumac, swelling, allergies, and bruises. However, its main use is for treatment of otitis, including otomycosis.

Ciprofloxacin/dexamethasone (Ciprodex) is an antibiotic/steroid combination medication. It contains the synthetic broad-spectrum antibacterial agent, ciprofloxacin hydrochloride (0.3%), combined with the anti-inflammatory corticosteroid, dexamethasone (0.1%), in a sterile, preserved suspension for otic use.

Ear drops are a form of topical medication for the ears used to treat infection, inflammation, impacted ear wax and local anesthesia. They are commonly used for short-term treatment and can be purchased with or without a prescription. Before using ear drops, refer to the package insert or consult a health professional for the amount of drops to use and the duration of treatment.
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis is the assessment that a particular [medical] condition is present while an indication is a reason for use. The opposite of an indication is a contraindication, a reason to withhold a certain medical treatment because the risks of treatment clearly outweigh the benefits.

The Center for Drug Evaluation and Research is a division of the U.S. Food and Drug Administration (FDA) that monitors most drugs as defined in the Food, Drug, and Cosmetic Act. Some biological products are also legally considered drugs, but they are covered by the Center for Biologics Evaluation and Research. The center reviews applications for brand name, generic, and over the counter pharmaceuticals, manages US current Good Manufacturing Practice (cGMP) regulations for pharmaceutical manufacturing, determines which medications require a medical prescription, monitors advertising of approved medications, and collects and analyzes safety data about pharmaceuticals that are already on the market.
Neomycin/polymyxin B/hydrocortisone, sold under the brand Otosporin among others, is a medication used to treat otitis externa and certain eye disorders. It consists of the antibiotics neomycin and polymyxin B, and the steroid hydrocortisone. It is used as an ear drop or eye drop.

Obetrol was the brand name of a drug combining several amphetamine salts indicated for the treatment of exogenous obesity. It was originally sold by the American company Obetrol Pharmaceuticals. Obetrol was a popular diet pill in America in the 1950s and 1960s.
Donnatal is a combination medication that provides natural belladonna alkaloids in a specific fixed ratio combined with phenobarbital to provide peripheral anticholinergic/antispasmodic action and mild sedation. Donnatal is manufactured for Concordia Pharmaceuticals by IriSys, LLC. It is available as tablets and 5 mL elixir. Active ingredients are listed as: phenobarbital (16.2 mg), hyoscyamine sulfate (0.1037 mg), atropine sulfate (0.0194 mg), and scopolamine hydrobromide (0.0065 mg). The latter two ingredients are found in plants of the family Solanaceae, such as belladonna.

President of the United States George W. Bush signed the Food and Drug Administration Amendments Act of 2007 (FDAAA) on September 27, 2007. This law reviewed, expanded, and reaffirmed several existing pieces of legislation regulating the FDA. These changes allow the FDA to perform more comprehensive reviews of potential new drugs and devices. It was sponsored by Reps. Joe Barton and Frank Pallone and passed unanimously by the Senate.
B&O Supprettes is the brand name for a prescription medication containing powdered opium and belladonna alkaloids in a suppository form. They are indicated for the treatment of moderate to severe pain from urethral spasm, and for extending the interval(s) between injections of opiates. The drug has various "off label" uses, including renal colic, intestinal cramps, tenesmus and diarrhea. They are also often prescribed after urinary bladder surgery. B&O Supprettes was unique in the United States because they were the only drug containing opium that is for suppository use sold in the US and, in fact, one of the very few medications that contains opium in any form in the US along with paregoric and opium tincture (laudanum).
Cetacaine is a topical anesthetic that contains the active ingredients benzocaine (14%), butamben (2%), and tetracaine hydrochloride (2%). Cetacaine also contains small amounts of benzalkonium chloride at 0.5% and 0.005% of cetyl dimethyl ethyl ammonium bromide all in a bland water-soluble base. Although Cetacaine has been widely used in the medical and dental fields, it has yet to be officially approved by the FDA. Cetacaine is produced by the company Cetylite Industries, Inc. and they provide Cetacaine in three forms: liquid, gel, and spray.

Finafloxacin (Xtoro) is a fluoroquinolone antibiotic. In the United States, it is approved by the Food and Drug Administration to treat acute otitis externa caused by the bacteria Pseudomonas aeruginosa and Staphylococcus aureus.

Janet Woodcock is an American physician and former Acting Commissioner of the U.S. Food and Drug Administration (FDA). She joined the FDA in 1986, and has held a number of senior leadership positions there, including terms as the Director of Center for Drug Evaluation and Research (CDER) from 1994 to 2004 and 2007 to 2021.

A cerumenolytic is an ear wax (cerumen) softening agent. Common cerumenolytics such as hydrogen peroxide and hydrogen peroxide - urea are topical preparations used to facilitate the removal of ear wax. Their side effects tend to be mild, including ear discomfort, transient loss of hearing, dizziness, and local irritation.
References
- ↑ "ANTIPYRINE WITH BENZOCAINE - OTIC". MedicineNet.com.
- 1 2 3 "Auralgan (Antipyrine, Benzocaine, and Glygerin dehydrated) Drug information. Uses, side effects". RxList.com Inc. Archived from the original on October 23, 2008. Retrieved 2008-11-10.
- ↑ "The new Auralgan is different from the older Auralgan" . Retrieved 2012-06-16.
- ↑ "Unapproved Prescription Ear Drop (Otic) Products: Not FDA Evaluated for Safety, Effectiveness and Quality". Food and Drug Administration . Retrieved 2015-10-27.
- ↑ "Drug Adverse Event Reporting Database" . Retrieved 2015-10-27.