Ketoprofen is one of the propionic acid class of nonsteroidal anti-inflammatory drugs (NSAID) with analgesic and antipyretic effects. It acts by inhibiting the body's production of prostaglandin.

Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.
An antipyretic is a substance that reduces fever. Antipyretics cause the hypothalamus to override a prostaglandin-induced increase in temperature. The body then works to lower the temperature, which results in a reduction in fever.

Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to relieve pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be taken orally or intravenously. It typically begins working within an hour.
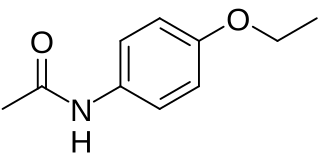
Phenacetin is a pain-relieving and fever-reducing drug, which was widely used following its introduction in 1887. It was withdrawn from medicinal use as dangerous from the 1970s.
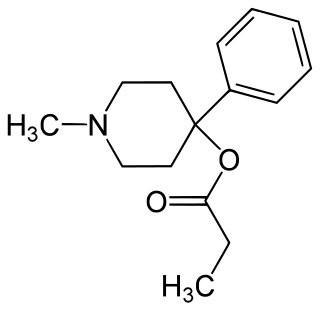
Desmethylprodine or 1-methyl-4-phenyl-4-propionoxypiperidine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. Desmethylprodine has been labeled by the DEA as a Schedule I drug in the United States. It is an analog of pethidine (meperidine) a Schedule II drug. Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. Unlike its derivative prodine, it does not exhibit optical isomerism. It was reported to have 30 times the activity of pethidine and a greater analgesic effect than morphine in rats, and it was demonstrated to cause central nervous system stimulation in mice.
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID). It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis. It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis. It is taken by mouth. Benefits are typically seen within an hour.

Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class used to relieve the symptoms of painful inflammatory conditions like arthritis. Piroxicam works by preventing the production of endogenous prostaglandins which are involved in the mediation of pain, stiffness, tenderness and swelling. The medicine is available as capsules, tablets and, in some countries, as a prescription-free gel 0.5%. It is also available in a betadex formulation, which allows a more rapid absorption of piroxicam from the digestive tract. Piroxicam is one of the few NSAIDs that can be given parenteral routes.

Ketorolac, sold under the brand name Toradol, Acular and Sprix, among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain. Specifically it is recommended for moderate to severe pain. Recommended duration of treatment is less than six days, and in Switzerland not more than seven days. It is used by mouth, by nose, by injection into a vein or muscle, and as eye drops. Effects begin within an hour and last for up to eight hours. Ketorolac also has antipyretic (fever-reducing) properties.

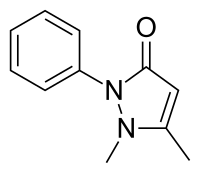
Metamizole or dipyrone is a painkiller, spasm reliever, and fever reliever drug. It is most commonly given by mouth or by intravenous infusion. It belongs to the ampyrone sulfonate family of medicines and was patented in 1922. Metamizole is marketed under various trade names. It was first used medically in Germany under the brand name "Novalgin", later becoming widely known in Slavic nations and India under the name "Analgin".
Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially in analgesics and dyes.
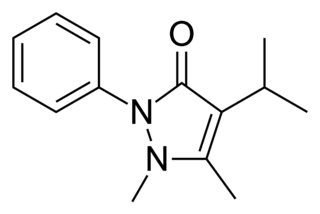
Propyphenazone is a derivative of phenazone with similar analgesic and antipyretic effects. Originally patented in 1931, propyphenazone is marketed as a combination formulation with paracetamol and caffeine for treatment of primary headache disorder.

Deracoxib is a nonsteroidal anti-inflammatory drug (NSAID) of the coxib class, used in dogs to treat pain associated with osteoarthritis, or to prevent pain following orthopedic or dental surgery. It is available as beef-flavored tablets.

Zomepirac is an orally effective nonsteroidal anti-inflammatory drug (NSAID) that has antipyretic actions. It was developed by McNeil Pharmaceutical, approved by the FDA in 1980, and sold as the sodium salt zomepirac sodium, under the brand name Zomax. Due to its clinical effectiveness, it was preferred by doctors in many situations and obtained a large share of the analgesics market; however, it was subsequently withdrawn in March 1983 due to its tendency to cause serious anaphylaxis in a small, but unpredictable, subset of the patient population.
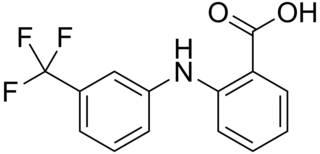
Flufenamic acid (FFA) is a member of the anthranilic acid derivatives class of nonsteroidal anti-inflammatory drugs (NSAIDs). Like other members of the class, it is a cyclooxygenase (COX) inhibitor, preventing the formation of prostaglandins. FFA is known to bind to and reduce the activity of prostaglandin F synthase and activate TRPC6.

Lornoxicam, also known as chlortenoxicam, is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class with analgesic, anti-inflammatory and antipyretic properties. It is available in oral and parenteral formulations.

Ludwig Knorr was a German chemist. Together with Carl Paal, he discovered the Paal–Knorr synthesis, and the Knorr quinoline synthesis and Knorr pyrrole synthesis are also named after him. The synthesis in 1883 of the analgesic drug antipyrine, now called phenazone, was a commercial success. Antipyrine was the first synthetic drug and the most widely used drug until it was replaced by Aspirin in the early 20th century.
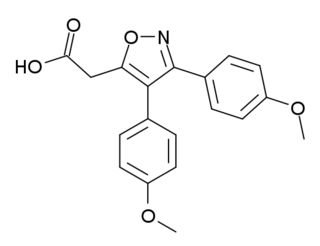
Mofezolac (INN), sold under the name Disopain in Japan, is a nonsteroidal anti-inflammatory drug (NSAID) used for its analgesic and anti-inflammatory actions. It is often prescribed for rheumatoid arthritis, lower back pain, frozen shoulder, and pain management after surgery or trauma. It is also being investigated for potential use in the treatment of neuroinflammation.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

Zaltoprofen is a nonsteroidal anti-inflammatory drug (NSAID) used as an analgesic, antipyretic, and anti-inflammatory agent. It is a selective COX-2 inhibitor and also inhibits bradykinin-induced pain responses without blocking bradykinin receptors.