Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.

Ertapenem, sold under the brand name Invanz, is a carbapenem antibiotic medication used for the treatment of infections of the abdomen, the lungs, the upper part of the female reproductive system, and the diabetic foot.

Troglitazone is an antidiabetic and anti-inflammatory drug, and a member of the drug class of the thiazolidinediones. It was prescribed for people with diabetes mellitus type 2.
A prodrug is a pharmacologically inactive medication or compound that, after intake, is metabolized into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug is absorbed, distributed, metabolized, and excreted (ADME).

Sufentanil, sold under the brand names Sufenta among others, is a synthetic opioid analgesic drug approximately 5 to 10 times as potent as its parent drug, fentanyl, and 500 to 1,000 times as potent as morphine. Structurally, sufentanil differs from fentanyl through the addition of a methoxymethyl group on the piperidine ring, and the replacement of the phenyl ring by thiophene. Sufentanil first was synthesized at Janssen Pharmaceutica in 1974.

Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive and local anaesthetic effects. The drug has been taken off the market in Europe and the US due to concerns of fatal overdoses and heart arrhythmias. It is still available in Australia, albeit with restrictions after an application by its manufacturer to review its proposed banning. Its onset of analgesia is said to be 20–30 minutes and peak effects are seen about 1.5–2.0 hours after oral administration.

Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class used to relieve the symptoms of painful inflammatory conditions like arthritis. Piroxicam works by preventing the production of endogenous prostaglandins which are involved in the mediation of pain, stiffness, tenderness and swelling. The medicine is available as capsules, tablets and, in some countries, as a prescription-free gel 0.5%. It is also available in a betadex formulation, which allows a more rapid absorption of piroxicam from the digestive tract. Piroxicam is one of the few NSAIDs that can be given parenteral routes.

Phenazone is an analgesic, antipyretic and anti-inflammatory drug. While it predates the term, it is often classified as a nonsteroidal anti-inflammatory drug (NSAID). Phenazone was one of the earliest synthetic medications — when it was patented in 1883, the only synthetic medical chemicals on the market were chloral hydrate, a sedative, trimethylamine, and iodol (tetraiodopyrrol), an early antiseptic. One of the earliest widely used analgesics and antipyretics, phenazone was gradually replaced in common use by other medications including phenacetin, aspirin, paracetamol and modern NSAIDs such as ibuprofen. However, it is still available in several countries either as an over-the-counter or prescribed drug.

Nefazodone, sold formerly under the brand names Serzone, Dutonin, and Nefadar among others, is an atypical antidepressant medication which is used in the treatment of depression and for other uses. Nefazodone is still available in the United States, but was withdrawn from other countries due to rare liver toxicity. The medication is taken by mouth.

Dosulepin, also known as dothiepin and sold under the brand name Prothiaden among others, is a tricyclic antidepressant (TCA) which is used in the treatment of depression. Dosulepin was once the most frequently prescribed antidepressant in the United Kingdom, but it is no longer widely used due to its relatively high toxicity in overdose without therapeutic advantages over other TCAs. It acts as a serotonin–norepinephrine reuptake inhibitor (SNRI) and also has other activities including antihistamine, antiadrenergic, antiserotonergic, anticholinergic, and sodium channel-blocking effects.

Oxandrolone is an androgen and synthetic anabolic steroid (AAS) medication to help promote weight gain in various situations, to help offset protein catabolism caused by long-term corticosteroid therapy, to support recovery from severe burns, to treat bone pain associated with osteoporosis, to aid in the development of girls with Turner syndrome, and for other indications. It is taken by mouth. It was sold under the brand names Oxandrin and Anavar, among others.

Nomifensine, sold under the brand names Merital and Alival, is a norepinephrine–dopamine reuptake inhibitor (NDRI), i.e. a drug that increases the amount of synaptic norepinephrine and dopamine available to receptors by blocking the dopamine and norepinephrine reuptake transporters. This is a mechanism of action shared by some recreational drugs like cocaine and the medication tametraline (see DRI). Research showed that the (S)-isomer is responsible for activity.

Biguanide is the organic compound with the formula HN(C(NH)NH2)2. It is a colorless solid that dissolves in water to give a highly basic solution. These solutions slowly hydrolyse to ammonia and urea.

Etretinate is a medication developed by Hoffmann–La Roche that was approved by the FDA in 1986 to treat severe psoriasis. It is a second-generation retinoid. It was subsequently removed from the Canadian market in 1996 and the United States market in 1998 due to the high risk of birth defects. It remains on the market in Japan as Tigason.

Indalpine, sold under the brand name Upstène, is a selective serotonin reuptake inhibitor (SSRI) that was briefly marketed as an antidepressant for treatment of depression. It was marketed in France and a few other European countries.

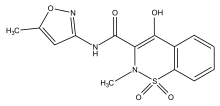
Lornoxicam, also known as chlortenoxicam, is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class with analgesic, anti-inflammatory and antipyretic properties. It is available in oral and parenteral formulations.
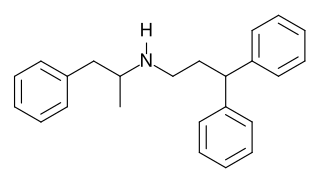
Prenylamine (Segontin) is a calcium channel blocker of the amphetamine chemical class that was used as a vasodilator in the treatment of angina pectoris.

Minaprine is a monoamine oxidase inhibitor antidepressant drug that was used in France for the treatment of depression until it was withdrawn from the market in 1996 because it caused convulsions.

Bucetin is an analgesic and antipyretic that is no longer marketed. Chemically, it is similar to phenacetin with which it shares the risk of carcinogenesis. Bucetin was withdrawn from use in 1986 due to renal toxicity.