
The prostaglandins (PG) are a group of physiologically active lipid compounds called eicosanoids having diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are derived enzymatically from the fatty acid arachidonic acid. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and of the prostanoid class of fatty acid derivatives.
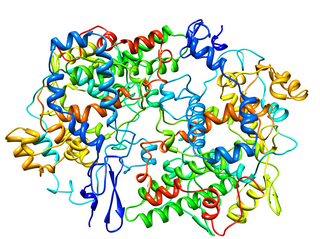
Cyclooxygenase (COX), officially known as prostaglandin-endoperoxide synthase (PTGS), is an enzyme that is responsible for formation of prostanoids, including thromboxane and prostaglandins such as prostacyclin, from arachidonic acid. A member of the animal-type heme peroxidase family, it is also known as prostaglandin G/H synthase. The specific reaction catalyzed is the conversion from arachidonic acid to prostaglandin H2 via a short-living prostaglandin G2 intermediate.
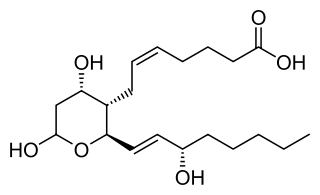
Thromboxane is a member of the family of lipids known as eicosanoids. The two major thromboxanes are thromboxane A2 and thromboxane B2. The distinguishing feature of thromboxanes is a 6-membered ether-containing ring.

Thromboxane A synthase 1 , also known as TBXAS1, is a cytochrome P450 enzyme that, in humans, is encoded by the TBXAS1 gene.

The thromboxane receptor (TP) also known as the prostanoid TP receptor is a protein that in humans is encoded by the TBXA2R gene, The thromboxane receptor is one among the five classes of prostanoid receptors and was the first eicosanoid receptor cloned. The TP receptor derives its name from its preferred endogenous ligand thromboxane A2.

Thromboxane A2 (TXA2) is a type of thromboxane that is produced by activated platelets during hemostasis and has prothrombotic properties: it stimulates activation of new platelets as well as increases platelet aggregation. This is achieved by activating the thromboxane receptor, which results in platelet-shape change, inside-out activation of integrins, and degranulation. Circulating fibrinogen binds these receptors on adjacent platelets, further strengthening the clot. Thromboxane A2 is also a known vasoconstrictor and is especially important during tissue injury and inflammation. It is also regarded as responsible for Prinzmetal's angina.

Picotamide is a platelet aggregation inhibitor. It works as a thromboxane synthase inhibitor and a thromboxane receptor inhibitor, the latter by modifying cellular responses to activation of the thromboxane receptor. Picotamide is licensed in Italy for the treatment of clinical arterial thrombosis and peripheral artery disease.

Prostaglandin D2 receptor 2 (DP2 or CRTH2) is a human protein encoded by the PTGDR2 gene and GPR44. DP2 has also been designated as CD294 (cluster of differentiation 294). It is a member of the class of prostaglandin receptors which bind with and respond to various prostaglandins. DP2 along with Prostaglandin DP1 receptor are receptors for prostaglandin D2 (PGD2). Activation of DP2 by PGD2 or other cognate receptor ligands has been associated with certain physiological and pathological responses, particularly those associated with allergy and inflammation, in animal models and certain human diseases.
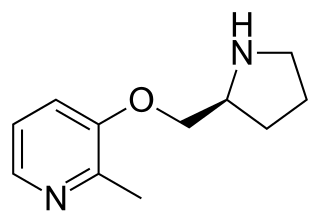
Pozanicline is a drug developed by Abbott, that has nootropic and neuroprotective effects. Animal studies suggested it useful for the treatment of ADHD and subsequent human trials have shown ABT-089 to be effective for this application. It binds with high affinity subtype-selective to the α4β2 nicotinic acetylcholine receptors and has partial agonism to the α6β2 subtype, but not the α7 and α3β4 subtypes familiar to nicotine. It has particularly low tendency to cause side effects compared to other drugs in the class, making it an exciting candidate for clinical development.
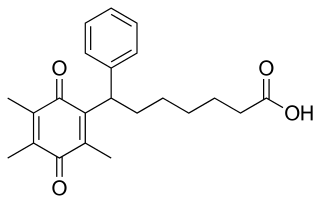
Seratrodast (development name, AA-2414; marketed originally as Bronica) is a thromboxane A2 (TXA2) receptor (TP receptor) antagonist used primarily in the treatment of asthma. It was the first TP receptor antagonist that was developed as an anti-asthmatic drug and received marketing approval in Japan in 1997. As of 2017 seratrodast was marketed as Bronica in Japan, and as Changnuo, Mai Xu Jia, Quan Kang Nuo in China.

Sarpogrelate is a drug which acts as an antagonist at the 5HT2A and 5-HT2B receptors. It blocks serotonin-induced platelet aggregation, and has applications in the treatment of many diseases including diabetes mellitus, Buerger's disease, Raynaud's disease, coronary artery disease, angina pectoris, and atherosclerosis.

Ifetroban is a potent and selective thromboxane receptor antagonist. It has been studied in animal models for the treatment of cancer metastasis, myocardial ischemia, hypertension, stroke, thrombosis, cardiomyopathy, and for its effects on platelets. Clinical trials are evaluating the therapeutic safety and efficacy of oral ifetroban capsules for the treatment of cancer metastasis, cardiovascular disease, aspirin exacerbated respiratory disease, systemic sclerosis, and Duchenne muscular dystrophy.

Elinogrel (INN, USAN) was an experimental antiplatelet drug acting as a P2Y12 inhibitor. Similarly to ticagrelor and in contrast to clopidogrel, elinogrel was a reversible inhibitor that acted fast and short (for about 12 hours), and it was not a prodrug but pharmacologically active itself. The substance was used in form of its potassium salt, intravenously for acute treatment and orally for long-term treatment. Development was terminated in 2012.
Milacemide (INN) is an MAO-B inhibitor and glycine prodrug. It has been studied for its effects on human memory and as a potential treatment for the symptoms of Alzheimer's disease. Early clinical trials did not show positive results however, and the drug is now abandoned and it is sold as a nonprescription drug or supplement. While milacemide is not an amino-acid, it acts similarly to glycine in the brain.

Epelsiban is an orally bioavailable drug which acts as a selective and potent oxytocin receptor antagonist. It was initially developed by GlaxoSmithKline (GSK) for the treatment of premature ejaculation in men and then as an agent to enhance embryo or blastocyst implantation in women undergoing embryo or blastocyst transfer associated with in vitro fertilization (IVF)., and was also investigated for use in the treatment of adenomyosis.
Thromboregulation is the series of mechanisms in how a primary clot is regulated. These mechanisms include, competitive inhibition or negative feedback. It includes primary hemostasis, which is the process of how blood platelets adhere to the endothelium of an injured blood vessel. Platelet aggregation is fundamental to repair vascular damage and the initiation of the blood thrombus formation. The elimination of clots is also part of thromboregulation. Failure in platelet clot regulation may cause hemorrhage or thrombosis. Substances called thromboregulators control every part of these events.

12-Hydroxyheptadecatrenoic acid is a 17 carbon metabolite of the 20 carbon polyunsaturated fatty acid, arachidonic acid. It was first detected and structurally defined by P. Wlodawer, Bengt I. Samuelsson, and M. Hamberg as a product of arachidonic acid metabolism made by microsomes isolated from sheep seminal vesicle glands and by intact human platelets. 12-HHT is less ambiguously termed 12-(S)-hydroxy-5Z,8E,10E-heptadecatrienoic acid to indicate the S stereoisomerism of its 12-hydroxyl residue and the Z, E, and E cis-trans isomerism of its three double bonds. The metabolite was for many years thought to be merely a biologically inactive byproduct of prostaglandin synthesis. More recent studies, however, have attached potentially important activity to it.

Setipiprant (INN; developmental code names ACT-129968, KYTH-105) is an investigational drug developed for the treatment of asthma and scalp hair loss. It was originally developed by Actelion and acts as a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2). The drug is being developed as a novel treatment for male pattern baldness by Allergan.
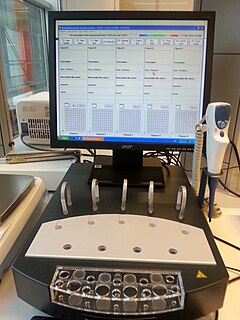
Multiplate multiple electrode aggregometry (MEA) is a test of platelet function in whole blood. The test can be used to diagnose platelet disorders, monitor antiplatelet therapy, and is also investigated as a potential predictor of transfusion requirements and bleeding risk in cardiac surgery.

Furegrelate, also known as 5-(3-pyridinylmethyl)benzofurancarboxylic acid, is a chemical compound with thromboxane enzyme inhibiting properties that was originally developed by Pharmacia Corporation as a drug to treat arrhythmias, ischaemic heart disorders, and thrombosis but was discontinued. It is commercially available in the form furegrelate sodium salt.


















