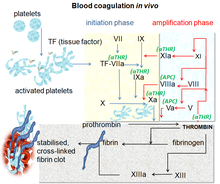
An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood.

Warfarin, sold under the brand name Coumadin among others, is an anticoagulant medication. While the drug is described as a "blood thinner", it does not reduce viscosity but rather prevents blood clots (thrombus) from forming (coagulating). Accordingly, it is commonly used to prevent deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Warfarin may sometimes be prescribed following ST-segment elevation myocardial infarctions (STEMI) and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously. It is a vitamin K antagonist.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and, in the treatment of venous thromboembolism, and the treatment of myocardial infarction.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.
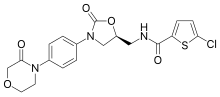
Ximelagatran is an anticoagulant that has been investigated extensively as a replacement for warfarin that would overcome the problematic dietary, drug interaction, and monitoring issues associated with warfarin therapy. In 2006, its manufacturer AstraZeneca announced that it would withdraw pending applications for marketing approval after reports of hepatotoxicity during trials, and discontinue its distribution in countries where the drug had been approved.

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth.

Phenprocoumon is a long-acting anticoagulant to be taken by mouth, and a coumarin derivative. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.

Dabigatran, sold under the brand name Pradaxa among others, is an anticoagulant used to treat and prevent blood clots and to prevent stroke in people with atrial fibrillation. Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots. It is used as an alternative to warfarin and does not require monitoring by blood tests. In a meta analysis of 7 different studies, there was no benefit of dabigatran over warfarin in preventing ischemic stroke; however, dabigatran were associated with a lower hazard for intracranial bleeding compared with warfarin, but also had a higher risk of gastrointestinal bleeding relative to warfarin. It is taken by mouth.
Prothrombin complex concentrate (PCC), also known as factor IX complex, sold under the brand name Kcentra among others, is a combination medication made up of blood clotting factors II, IX, and X(3-factor PCC) or, when also containing factor VII as does Kcentra, 4-factor PCC. It is used to treat and prevent bleeding in hemophilia B if pure factor IX is not available. It may also be used for reversal of warfarin therapy. It is given by slow injection into a vein. Another product, activated prothrombin complex concentrate or FEIBA, may be used for acquired hemophilia.
Direct thrombin inhibitors (DTIs) are a class of medication that act as anticoagulants by directly inhibiting the enzyme thrombin. Some are in clinical use, while others are undergoing clinical development. Several members of the class are expected to replace heparin and warfarin in various clinical scenarios.
Hypercoagulability in pregnancy is the propensity of pregnant women to develop thrombosis. Pregnancy itself is a factor of hypercoagulability, as a physiologically adaptive mechanism to prevent post partum bleeding. However, when combined with an additional underlying hypercoagulable states, the risk of thrombosis or embolism may become substantial.
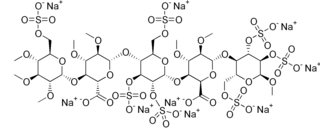
Idraparinux sodium is an anticoagulant medication in development by Sanofi-Aventis.

Vitamin K antagonists (VKA) are a group of substances that reduce blood clotting by reducing the action of vitamin K. The term "vitamin K antagonist" is technically a misnomer, as the drugs do not directly antagonize the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K. Vitamin K antagonists (VKAs) have been the mainstay of anticoagulation therapy for more than 50 years.
The management of atrial fibrillation (AF) is focused on preventing temporary circulatory instability, stroke and other ischemic events. Control of heart rate and rhythm are principally used to achieve the former, while anticoagulation may be employed to decrease the risk of stroke. Within the context of stroke, the discipline may be referred to as stroke prevention in atrial fibrillation (SPAF). In emergencies, when circulatory collapse is imminent due to uncontrolled rapid heart rate, immediate cardioversion may be indicated.

Edoxaban, sold under the brand name Lixiana among others, is an anticoagulant medication and a direct factor Xa inhibitor. It is taken by mouth.

Apixaban, sold under the brand name Eliquis, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa. It is used as an alternative to warfarin to prevent blood clots following hip or knee replacement and in those with a history of prior clots. and does not require monitoring by blood tests or dietary restrictions. It is taken by mouth.

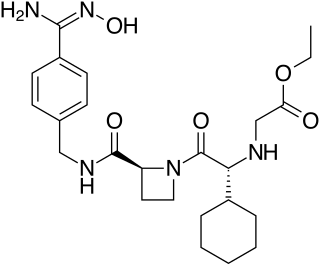
Darexaban (YM150) is a direct inhibitor of factor Xa created by Astellas Pharma. It is an experimental drug that acts as an anticoagulant and antithrombotic to prevent venous thromboembolism after a major orthopaedic surgery, stroke in patients with atrial fibrillation and possibly ischemic events in acute coronary syndrome. It is used in form of the maleate. The development of darexaban was discontinued in September 2011.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.
The SAMe-TT2R2 score is a clinical prediction rule to predict the quality of vitamin K antagonist anticoagulation therapy as measured by time in therapeutic INR range (TTR) (VKA e.g. warfarin). It has been suggested that it can aid in the medical decision making between VKAs and new oral anticoagulant/non-VKA oral anticoagulant (NOAC e.g. dabigatran, rivaroxaban, apixaban or edoxaban) in patients with atrial fibrillation (AF). This score can be used with patients with ≥1 additional stroke risk factors using the CHA2DS2-VASc score, where oral anticoagulation is recommended or should be considered.
Four drugs from the class of direct Xa inhibitors are marketed worldwide. Rivaroxaban (Xarelto) was the first approved FXa inhibitor to become commercially available in Europe and Canada in 2008. The second one was apixaban (Eliquis), approved in Europe in 2011 and in the United States in 2012. The third one edoxaban was approved in Japan in 2011 and in Europe and the US in 2015. Betrixaban (Bevyxxa) was approved in the US in 2017.