
Aspirin is the genericized trademark for acetylsalicylic acid (ASA), a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and inflammation, and as an antithrombotic. Specific inflammatory conditions that aspirin is used to treat include Kawasaki disease, pericarditis, and rheumatic fever.

Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.

Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to relieve pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be taken orally or intravenously. It typically begins working within an hour.
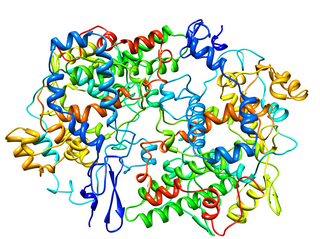
Cyclooxygenase (COX), officially known as prostaglandin-endoperoxide synthase (PTGS), is an enzyme that is responsible for biosynthesis of prostanoids, including thromboxane and prostaglandins such as prostacyclin, from arachidonic acid. A member of the animal-type heme peroxidase family, it is also known as prostaglandin G/H synthase. The specific reaction catalyzed is the conversion from arachidonic acid to prostaglandin H2 via a short-living prostaglandin G2 intermediate.

Naproxen, sold under the brand name Aleve among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain, menstrual cramps, and inflammatory diseases such as rheumatoid arthritis, gout and fever. It is taken orally. It is available in immediate and delayed release formulations. Onset of effects is within an hour and lasts for up to twelve hours. Naproxen is also available in salt form, naproxen sodium, which has better solubility when taken orally.

Rofecoxib is a COX-2-selective nonsteroidal anti-inflammatory drug (NSAID). It was marketed by Merck & Co. to treat osteoarthritis, rheumatoid arthritis, juvenile rheumatoid arthritis, acute pain conditions, migraine, and dysmenorrhea. Rofecoxib was approved in the US by the US Food and Drug Administration (FDA) in May 1999, and was marketed under the brand names Vioxx, Ceoxx, and Ceeoxx. Rofecoxib was available by prescription in both tablets and as an oral suspension.
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID). It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis. It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis. It is taken by mouth. Benefits are typically seen within an hour.
Cyclooxygenase-2 inhibitors, also known as coxibs, are a type of nonsteroidal anti-inflammatory drug (NSAID) that directly target cyclooxygenase-2 (COX-2), an enzyme responsible for inflammation and pain. Targeting selectivity for COX-2 reduces the risk of peptic ulceration and is the main feature of celecoxib, rofecoxib, and other members of this drug class.

Indometacin, also known as indomethacin, is a nonsteroidal anti-inflammatory drug (NSAID) commonly used as a prescription medication to reduce fever, pain, stiffness, and swelling from inflammation. It works by inhibiting the production of prostaglandins, endogenous signaling molecules known to cause these symptoms. It does this by inhibiting cyclooxygenase, an enzyme that catalyzes the production of prostaglandins.
Etoricoxib, sold under the brand name Arcoxia, is a selective COX-2 inhibitor developed and commercialized by Merck. It is approved in 63 countries worldwide as of 2007, except the United States where the Food and Drug Administration sent a Non Approvable Letter to Merck and required them to provide additional data.

Nimesulide is a nonsteroidal anti-inflammatory drug (NSAID) with pain medication and fever reducing properties. Its approved indications are the treatment of acute pain, the symptomatic treatment of osteoarthritis, and primary dysmenorrhoea in adolescents and adults above 12 years old.
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis. It is taken by mouth or given by injection into a vein. It is recommended that it be used for as short a period as possible and at a low dose.

Nabumetone, sold under the brand name Relafen among others, is a nonsteroidal anti-inflammatory drug (NSAID). Nabumetone was developed by Beecham and first received regulatory approval in 1991.
A prostaglandin antagonist is a hormone antagonist acting upon one or more prostaglandins, a subclass of eicosanoid compounds which function as signaling molecules in numerous types of animal tissues.

Nicox S.A. is a French ophthalmology company developing treatments to maintain vision and improve ocular health. Nicox is headquartered in Sophia Antipolis, France, and its Chairman and CEO is Michele Garufi.
COX-inhibiting nitric oxide donators (CINODs), also known as NO-NSAIDs, are a new class of nonsteroidal anti-inflammatory drug (NSAID) developed with the intention of providing greater safety than existing NSAIDs.

Licofelone is a dual COX/LOX inhibitor that was studied in clinical trials as a treatment for osteoarthritis and which was under development by Merckle GmbH with partners Alfa Wassermann and Lacer.
Cyclooxygenases are enzymes that take part in a complex biosynthetic cascade that results in the conversion of polyunsaturated fatty acids to prostaglandins and thromboxane(s). Their main role is to catalyze the transformation of arachidonic acid into the intermediate prostaglandin H2, which is the precursor of a variety of prostanoids with diverse and potent biological actions. Cyclooxygenases have two main isoforms that are called COX-1 and COX-2. COX-1 is responsible for the synthesis of prostaglandin and thromboxane in many types of cells, including the gastro-intestinal tract and blood platelets. COX-2 plays a major role in prostaglandin biosynthesis in inflammatory cells and in the central nervous system. Prostaglandin synthesis in these sites is a key factor in the development of inflammation and hyperalgesia. COX-2 inhibitors have analgesic and anti-inflammatory activity by blocking the transformation of arachidonic acid into prostaglandin H2 selectively.

Otenaproxesul is a analgesic and anti-inflammatory drug being developed by Antibe Therapeutics. An NSAID structurally derived from naproxen, in 2016 it received approval to commence phase II clinical trials as a treatment for osteoarthritis after completing phase I clinical trials in 2015. In 2018, the drug completed trials for gastrointestinal safety, and in 2020 completed phase IIb trials on efficacy of pain reduction. Initial phase III clinical trials in 2021 failed to meet the necessary criteria to advance to the next phase.
Prostaglandin inhibitors are drugs that inhibit the synthesis of prostaglandin in human body. There are various types of prostaglandins responsible for different physiological reactions such as maintaining the blood flow in stomach and kidney, regulating the contraction of involuntary muscles and blood vessels, and act as a mediator of inflammation and pain. Cyclooxygenase (COX) and Phospholipase A2 are the major enzymes involved in prostaglandin production, and they are the drug targets for prostaglandin inhibitors. There are mainly 2 classes of prostaglandin inhibitors, namely non- steroidal anti- inflammatory drugs (NSAIDs) and glucocorticoids. In the following sections, the medical uses, side effects, contraindications, toxicity and the pharmacology of these prostaglandin inhibitors will be discussed.















