Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.

Ritonavir, a protease inhibitor sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Often a low dose is used with other protease inhibitors. It may also be used in combination with other medications for hepatitis C. It is taken by mouth. The tablets of ritonavir are not bioequivalent to capsules as tablets may result in higher peak plasma concentrations.

Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme.
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the CYP2D6 gene. CYP2D6 is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra.

Methoxsalen, sold under the brand name Oxsoralen among others, is a medication used to treat psoriasis, eczema, vitiligo, and some cutaneous lymphomas in conjunction with exposing the skin to ultraviolet (UVA) light from lamps or sunlight. Methoxsalen modifies the way skin cells receive the UVA radiation, allegedly clearing up the disease. Levels of individual patient PUVA exposure were originally determined using the Fitzpatrick scale. The scale was developed after patients demonstrated symptoms of phototoxicity after oral ingestion of methoxsalen followed by PUVA therapy. Chemically, methoxsalen belongs to a class of organic natural molecules known as furanocoumarins. They consist of coumarin annulated with furan. It can also be injected and used topically.

Cilostazol, sold under the brand name Pletal among others, is a medication used to help the symptoms of intermittent claudication in peripheral vascular disease. If no improvement is seen after 3 months, stopping the medication is reasonable. It may also be used to prevent stroke. It is taken by mouth.

Psoralen is the parent compound in a family of naturally occurring organic compounds known as the linear furanocoumarins. It is structurally related to coumarin by the addition of a fused furan ring, and may be considered as a derivative of umbelliferone. Psoralen occurs naturally in the seeds of Psoralea corylifolia, as well as in the common fig, celery, parsley, West Indian satinwood, and in all citrus fruits. It is widely used in PUVA treatment for psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma; these applications are typically through the use of medications such as Methoxsalen. Many furanocoumarins are extremely toxic to fish, and some are deposited in streams in Indonesia to catch fish.

Naringin is a flavanone-7-O-glycoside between the flavanone naringenin and the disaccharide neohesperidose. The flavonoid naringin occurs naturally in citrus fruits, especially in grapefruit, where naringin is responsible for the fruit's bitter taste. In commercial grapefruit juice production, the enzyme naringinase can be used to remove the bitterness created by naringin. In humans naringin is metabolized to the aglycone naringenin by naringinase present in the gut.
Cytochrome P450 1A2, a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the human body. In humans, the CYP1A2 enzyme is encoded by the CYP1A2 gene.
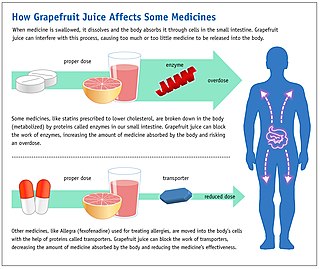
Some fruit juices and fruits can interact with numerous drugs, in many cases causing adverse effects. The effect was first discovered accidentally, when a test of drug interactions with alcohol used grapefruit juice to hide the taste of the ethanol.

Cytochrome P450 2C19 is an enzyme protein. It is a member of the CYP2C subfamily of the cytochrome P450 mixed-function oxidase system. This subfamily includes enzymes that catalyze metabolism of xenobiotics, including some proton pump inhibitors and antiepileptic drugs. In humans, it is the CYP2C19 gene that encodes the CYP2C19 protein. CYP2C19 is a liver enzyme that acts on at least 10% of drugs in current clinical use, most notably the antiplatelet treatment clopidogrel (Plavix), drugs that treat pain associated with ulcers, such as omeprazole, antiseizure drugs such as mephenytoin, the antimalarial proguanil, and the anxiolytic diazepam.
Synergistic enhancers of antiretrovirals usually do not possess any antiretroviral properties alone, but when they are taken concurrently with antiretroviral drugs they enhance the effect of that drug.
The erythromycin breath test (ERMBT) is a method used to measure metabolism (oxidation and elimination from the system) by a part of the cytochrome P450 system. Erythromycin produces 14CO2, and this 14CO2 can be measured to study drugs that interact with the cytochrome P450 system. Erythromycin is tagged with carbon-14 and given as an intravenous injection; after 20 minutes the subject blows up a balloon and the carbon dioxide exhaled that is tagged with carbon-14 shows the activity of the CYP3A4 isoenzyme on the erythromycin. ERMBT can be used to determine how drugs that the CYP3A4 isoenzyme metabolizes will function in a given individual.

The furanocoumarins, or furocoumarins, are a class of organic chemical compounds produced by a variety of plants. Most of the plant species found to contain furanocoumarins belong to a handful of plant families. The families Apiaceae and Rutaceae include the largest numbers of plant species that contain furanocoumarins. The families Moraceae and Fabaceae include a few, widely distributed plant species that contain furanocoumarins.
In enzymology, a psoralen synthase (EC 1.14.13.102) is an enzyme that catalyzes the chemical reaction

In enzymology, a sterol 14-demethylase (EC 1.14.13.70) is an enzyme of the Cytochrome P450 (CYP) superfamily. It is any member of the CYP51 family. It catalyzes a chemical reaction such as:

Cobicistat, sold under the brand name Tybost, is a medication for use in the treatment of human immunodeficiency virus infection (HIV/AIDS). Its major mechanism of action is through the inhibition of human CYP3A proteins.

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarine.

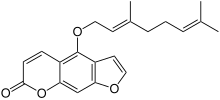
6',7'-Dihydroxybergamottin is a natural furanocoumarin found in pomelos, grapefruits, and sour oranges, in both the peel and the pulp. Along with the chemically related compound bergamottin, it is believed to be responsible for a number of grapefruit–drug interactions, in which the consumption of citrus containing one or both of these compounds affects the metabolism of a variety of pharmaceutical drugs.
Cytochrome P450 oxidoreductase deficiency (PORD) is a rare disease and inborn error of metabolism caused by deficiency of cytochrome P450 oxidoreductase (POR). POR is a 2-flavin protein that is responsible for the transfer of electrons from NADPH to all 50 microsomal cytochrome P450 (CYP450) enzymes. This includes the steroidogenic enzymes CYP17A1 (17α-hydroxylase/17,20-lyase), CYP19A1 (aromatase), and CYP21A2 (21-hydroxylase); CYP26B1 ; and the hepatic drug-metabolizing CYP450 enzymes, among many other CYP450 enzymes. Symptoms of severe forms of PORD include ambiguous genitalia in males and females, congenital adrenal hyperplasia, cortisol deficiency, and Antley–Bixler skeletal malformation syndrome (ABS), while symptoms of mild forms include polycystic ovary syndrome in women and hypogonadism in men. Maternal virilization also occurs in severe forms, due to aromatase deficiency in the placenta. Virilization of female infants in PORD may also be caused by alternative biosynthesis of 5α-dihydrotestosterone via the so-called "androgen backdoor pathway". The ABS component of severe forms of PORD is probably caused by CYP26B1 deficiency, which results in retinoic acid excess and defects during skeletal embryogenesis. All forms of PORD in humans are likely partial, as POR knockout in mice results in death during prenatal development.