Related Research Articles

Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin molybdaenum, which is based on Ancient Greek Μόλυβδος molybdos, meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.

In chemistry, many authors consider an organic compound to be any chemical compound that contains carbon-hydrogen or carbon-carbon bonds, however, some authors consider an organic compound to be any chemical compound that contains carbon. The definition of "organic" versus "inorganic" varies from author to author, and is a topic of debate. For example, methane is considered organic, but whether some other carbon-containing compounds are organic or inorganic varies from author to author, for example halides of carbon without carbon-hydrogen and carbon-carbon bonds, and certain compounds of carbon with nitrogen and oxygen.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
Nitrogenases are enzymes (EC 1.18.6.1EC 1.19.6.1) that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules (nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase.
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins.
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, forming dinitrosyl iron complexes. In most Fe–S proteins, the terminal ligands on Fe are thiolate, but exceptions exist.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopterin itself contains no molybdenum; rather, this is the name of the ligand that will bind the active metal. After molybdopterin is eventually complexed with molybdenum, the complete ligand is usually called molybdenum cofactor.
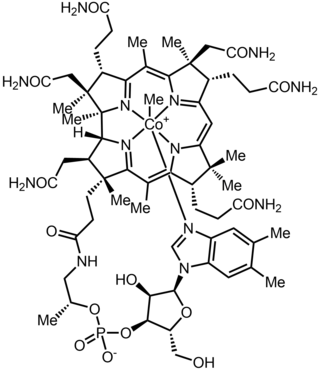
Methylcobalamin (mecobalamin, MeCbl, or MeB12) is a cobalamin, a form of vitamin B12. It differs from cyanocobalamin in that the cyano group at the cobalt is replaced with a methyl group. Methylcobalamin features an octahedral cobalt(III) centre and can be obtained as bright red crystals. From the perspective of coordination chemistry, methylcobalamin is notable as a rare example of a compound that contains metal–alkyl bonds. Nickel–methyl intermediates have been proposed for the final step of methanogenesis.
A hydrogenase mimic or bio-mimetic is an enzyme mimic of hydrogenases.
In enzymology, carbon monoxide dehydrogenase (CODH) (EC 1.2.7.4) is an enzyme that catalyzes the chemical reaction

The 5,10-methenyltetrahydromethanopterin hydrogenase, the so-called iron-sulfur cluster-free hydrogenase, is an enzyme found in methanogenic archea such as Methanothermobacter marburgensis. It was discovered and first characterized by the Thauer group at the Max Planck Institute in Marburg. Hydrogenases are enzymes that either reduce protons or oxidize molecular dihydrogen.

Organocobalt chemistry is the chemistry of organometallic compounds containing a carbon to cobalt chemical bond. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds exhibit useful catalytic properties, the preeminent example being dicobalt octacarbonyl.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.
Iron–nickel (Fe–Ni) clusters are metal clusters consisting of iron and nickel, i.e. Fe–Ni structures displaying polyhedral frameworks held together by two or more metal–metal bonds per metal atom, where the metal atoms are located at the vertices of closed, triangulated polyhedra.
Marcetta York Darensbourg is an American inorganic chemist. She is a Distinguished Professor of Chemistry at Texas A&M University. Her current work focuses on iron hydrogenases and iron nitrosyl complexes.

Acetyl-CoA synthase (ACS), not to be confused with Acetyl-CoA synthetase or Acetate-CoA ligase, is a nickel-containing enzyme involved in the metabolic processes of cells. Together with Carbon monoxide dehydrogenase (CODH), it forms the bifunctional enzyme Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase (ACS/CODH) found in anaerobic organisms such as archaea and bacteria. The ACS/CODH enzyme works primarily through the Wood–Ljungdahl pathway which converts carbon dioxide to Acetyl-CoA. The recommended name for this enzyme is CO-methylating acetyl-CoA synthase.
Evolution of metal ions in biological systems refers to the incorporation of metallic ions into living organisms and how it has changed over time. Metal ions have been associated with biological systems for billions of years, but only in the last century have scientists began to truly appreciate the scale of their influence. Major and minor metal ions have become aligned with living organisms through the interplay of biogeochemical weathering and metabolic pathways involving the products of that weathering. The associated complexes have evolved over time.

Transition metal acyl complexes describes organometallic complexes containing one or more acyl (RCO) ligands. Such compounds occur as transient intermediates in many industrially useful reactions, especially carbonylations.
References
- ↑ Hodgkin DG, Pickworth J, Robertson JH, Trueblood KN, Prosen RJ, White JG (August 1955). "The crystal structure of the hexacarboxylic acid derived from B12 and the molecular structure of the vitamin". Nature. 176 (4477): 325–328. Bibcode:1955Natur.176..325H. doi:10.1038/176325a0. PMID 13253565. S2CID 4220926.
- ↑ Sigel A, Sigel H, Sigel RK, eds. (2009). Metal-carbon bonds in enzymes and cofactors. Metal Ions in Life Sciences. Vol. 6. Royal Society of Chemistry. ISBN 978-1-84755-915-9.
- ↑ Linck RC, Rauchfuss TB (2005). "Synthetic Models for Bioorganometallic Reaction Centers". In Jaouen G (ed.). Bioorganometallics: Biomolecules, Labeling, Medicine. Weinheim: Wiley-VCH. pp. 403–435. doi:10.1002/3527607692.ch12. ISBN 978-3-527-30990-0.
- ↑ Jaouen G (2006). Bioorganometallics : biomolecules, labeling, medicine. Weinheim: Wiley-VCH. ISBN 3527607692. OCLC 85821090.
- ↑ Cammack R, Frey M, Robson R (2001). Hydrogen as a Fuel: Learning from Nature. London: Taylor & Francis. ISBN 978-0-415-24242-4.
- ↑ Volbeda A, Fontecilla-Camps JC (2003). "The Active Site and Catalytic Mechanism of NiFe Hydrogenases". Dalton Transactions (21): 4030–4038. doi:10.1039/B304316A.
- ↑ Hiromoto T, Ataka K, Pilak O, Vogt S, Stagni MS, Meyer-Klaucke W, et al. (February 2009). "The crystal structure of C176A mutated [Fe]-hydrogenase suggests an acyl-iron ligation in the active site iron complex". FEBS Letters. 583 (3): 585–590. doi:10.1016/j.febslet.2009.01.017. PMID 19162018. S2CID 1055079.
- ↑ Schaupp S, Arriaza-Gallardo FJ, Pan HJ, Kahnt J, Angelidou G, Paczia N, et al. (May 2022). "In Vitro Biosynthesis of the [Fe]-Hydrogenase Cofactor Verifies the Proposed Biosynthetic Precursors". Angewandte Chemie. 61 (22): e202200994. doi:10.1002/anie.202200994. PMC 9314073 . PMID 35286742.
- ↑ Spatzal T, Aksoyoglu M, Zhang L, Andrade SL, Schleicher E, Weber S, et al. (November 2011). "Evidence for interstitial carbon in nitrogenase FeMo cofactor". Science. 334 (6058): 940. Bibcode:2011Sci...334..940S. doi:10.1126/science.1214025. PMC 3268367 . PMID 22096190.
- ↑ Lancaster KM, Roemelt M, Ettenhuber P, Hu Y, Ribbe MW, Neese F, et al. (November 2011). "X-ray emission spectroscopy evidences a central carbon in the nitrogenase iron-molybdenum cofactor". Science. 334 (6058): 974–977. Bibcode:2011Sci...334..974L. doi:10.1126/science.1206445. PMC 3800678 . PMID 22096198.
- ↑ Rankin JA, Mauban RC, Fellner M, Desguin B, McCracken J, Hu J, et al. (June 2018). "Lactate Racemase Nickel-Pincer Cofactor Operates by a Proton-Coupled Hydride Transfer Mechanism". Biochemistry. 57 (23): 3244–3251. doi:10.1021/acs.biochem.8b00100. OSTI 1502215. PMID 29489337.
- ↑ Duan X, Block E, Li Z, Connelly T, Zhang J, Huang Z, et al. (February 2012). "Crucial role of copper in detection of metal-coordinating odorants". Proceedings of the National Academy of Sciences of the United States of America. 109 (9): 3492–3497. Bibcode:2012PNAS..109.3492D. doi: 10.1073/pnas.1111297109 . PMC 3295281 . PMID 22328155.