Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

Infrared spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers, symbol μm, which are related to the wavenumber in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. Two-dimensional IR is also possible as discussed below.

Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.

Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.

Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relatively inexpensive and easily implemented, this methodology is widely used in diverse applied and fundamental applications. The only requirement is that the sample absorb in the UV-Vis region, i.e. be a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy. Parameters of interest, besides the wavelength of measurement, are absorbance (A) or transmittance (%T) or reflectance (%R), and its change with time.

In electrochemistry, cyclic voltammetry (CV) is a type of potentiodynamic measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.

Surface-enhanced Raman spectroscopy or surface-enhanced Raman scattering (SERS) is a surface-sensitive technique that enhances Raman scattering by molecules adsorbed on rough metal surfaces or by nanostructures such as plasmonic-magnetic silica nanotubes. The enhancement factor can be as much as 1010 to 1011, which means the technique may detect single molecules.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.

Instrumental analysis is a field of analytical chemistry that investigates analytes using scientific instruments.
Scanning electrochemical microscopy (SECM) is a technique within the broader class of scanning probe microscopy (SPM) that is used to measure the local electrochemical behavior of liquid/solid, liquid/gas and liquid/liquid interfaces. Initial characterization of the technique was credited to University of Texas electrochemist, Allen J. Bard, in 1989. Since then, the theoretical underpinnings have matured to allow widespread use of the technique in chemistry, biology and materials science. Spatially resolved electrochemical signals can be acquired by measuring the current at an ultramicroelectrode (UME) tip as a function of precise tip position over a substrate region of interest. Interpretation of the SECM signal is based on the concept of diffusion-limited current. Two-dimensional raster scan information can be compiled to generate images of surface reactivity and chemical kinetics.
Operando spectroscopy is an analytical methodology wherein the spectroscopic characterization of materials undergoing reaction is coupled simultaneously with measurement of catalytic activity and selectivity. The primary concern of this methodology is to establish structure-reactivity/selectivity relationships of catalysts and thereby yield information about mechanisms. Other uses include those in engineering improvements to existing catalytic materials and processes and in developing new ones.
In electrochemistry, protein film voltammetry is a technique for examining the behavior of proteins immobilized on an electrode. The technique is applicable to proteins and enzymes that engage in electron transfer reactions and it is part of the methods available to study enzyme kinetics.

Infrared photodissociation (IRPD) spectroscopy uses infrared radiation to break bonds in, often ionic, molecules (photodissociation), within a mass spectrometer. In combination with post-ionization, this technique can also be used for neutral species. IRPD spectroscopy has been shown to use electron ionization, corona discharge, and electrospray ionization to obtain spectra of volatile and nonvolatile compounds. Ionized gases trapped in a mass spectrometer can be studied without the need of a solvent as in infrared spectroscopy.
Single-Entity Electrochemistry (SEE) refers to the electroanalysis of an individual unit of interest. A unique feature of SEE is that it unifies multiple different branches of electrochemistry. Single-Entity Electrochemistry pushes the bounds of the field as it can measure entities on a scale of 100 microns to angstroms. Single-Entity Electrochemistry is important because it gives the ability to view how a single molecule, or cell, or "thing" affects the bulk response, and thus the chemistry that might have gone unknown otherwise. The ability to monitor the movement of one electron or ion from one unit to another is valuable, as many vital reactions and mechanisms undergo this process. Electrochemistry is well suited for this measurement due to its incredible sensitivity. Single-Entity Electrochemistry can be used to investigate nanoparticles, wires, vesicles, nanobubbles, nanotubes, cells, and viruses, and other small molecules and ions. Single-entity electrochemistry has been successfully used to determine the size distribution of particles as well as the number of particles present inside a vesicle or other similar structures
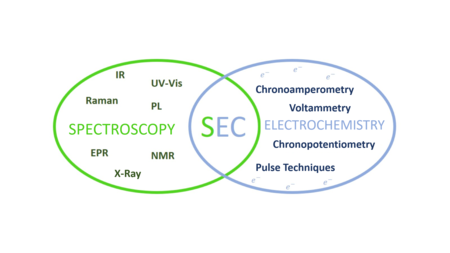
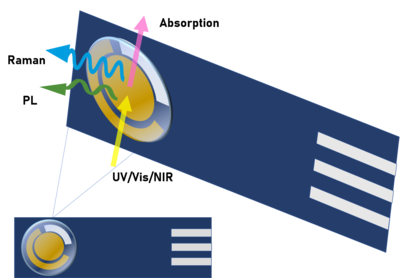
Ultraviolet-visible (UV-Vis) absorption spectroelectrochemistry (SEC) is a multiresponse technique that analyzes the evolution of the absorption spectra in UV-Vis regions during an electrode process. This technique provides information from an electrochemical and spectroscopic point of view. In this way, it enables a better perception about the chemical system of interest. On one hand, molecular information related to the electronic levels of the molecules is obtained from the evolution of the spectra. On the other hand, kinetic and thermodynamic information of the processes is obtained from the electrochemical signal.
Raman spectroelectrochemistry (Raman-SEC) is a technique that studies the inelastic scattering or Raman scattering of monochromatic light related to chemical compounds involved in an electrode process. This technique provides information about vibrational energy transitions of molecules, using a monochromatic light source, usually from a laser that belongs to the UV, Vis or NIR region. Raman spectroelectrochemistry provides specific information about structural changes, composition and orientation of the molecules on the electrode surface involved in an electrochemical reaction, being the Raman spectra registered a real fingerprint of the compounds.
In physics, electrical engineering and materials science, electromaterials are the set of materials which store, controllably convert, exchange and conduct electrically charged particles. The term electromaterial can refer to any electronically or ionically active material. While this definition is quite broad, the term is typically used in the context of properties and/or applications in which atomic electronic transition is pertinent. The word electromaterials is a compound form of the Ancient Greek term, ἤλεκτρον ēlektron, "Amber", and the Latin term, materia, "Matter".