
Gangrene is a type of tissue death caused by a lack of blood supply. Symptoms may include a change in skin color to red or black, numbness, swelling, pain, skin breakdown, and coolness. The feet and hands are most commonly affected. If the gangrene is caused by an infectious agent, it may present with a fever or sepsis.

Necrotizing fasciitis (NF), also known as flesh-eating disease, is a bacterial infection that results in the death of parts of the body's soft tissue. It is a severe disease of sudden onset that spreads rapidly. Symptoms usually include red or purple skin in the affected area, severe pain, fever, and vomiting. The most commonly affected areas are the limbs and perineum.

Clostridium perfringens is a Gram-positive, rod-shaped, anaerobic, spore-forming pathogenic bacterium of the genus Clostridium. C. perfringens is ever-present in nature and can be found as a normal component of decaying vegetation, marine sediment, the intestinal tract of humans and other vertebrates, insects, and soil. It has the shortest reported generation time of any organism at 6.3 minutes in thioglycolate medium.
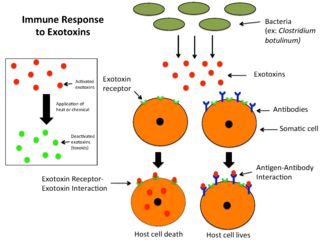
An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.
Enteritis is inflammation of the small intestine. It is most commonly caused by food or drink contaminated with pathogenic microbes, such as Serratia, but may have other causes such as NSAIDs, radiation therapy as well as autoimmune conditions like Crohn's disease and celiac disease. Symptoms include abdominal pain, cramping, diarrhea, dehydration, and fever. Related diseases of the gastrointestinal system include inflammation of the stomach and large intestine.

An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. They can be chromosomally or plasmid encoded. They are heat labile (>60⁰), of low molecular weight and water-soluble. Enterotoxins are frequently cytotoxic and kill cells by altering the apical membrane permeability of the mucosal (epithelial) cells of the intestinal wall. They are mostly pore-forming toxins, secreted by bacteria, that assemble to form pores in cell membranes. This causes the cells to die.

Gas gangrene is a bacterial infection that produces tissue gas in gangrene. This deadly form of gangrene usually is caused by Clostridium perfringens bacteria. About 1,000 cases of gas gangrene are reported yearly in the United States.
Lecithinase is a type of phospholipase that acts upon lecithin.
Cytolysin refers to the substance secreted by microorganisms, plants or animals that is specifically toxic to individual cells, in many cases causing their dissolution through lysis. Cytolysins that have a specific action for certain cells are named accordingly. For instance, the cytolysins responsible for the destruction of red blood cells, thereby liberating hemoglobins, are named hemolysins, and so on. Cytolysins may be involved in immunity as well as in venoms.
Clostridial necrotizing enteritis (CNE) is a severe and potentially fatal type of food poisoning caused by a β-toxin of Clostridium perfringens, Type C. It occurs in some developing regions, particularly in New Guinea, where it is known as pig-bel. The disease was also documented in Germany following World War II, where it was called Darmbrand (literally "bowel fire," or bowel necrosis). The toxin is normally inactivated by certain proteolytic enzymes and by normal cooking, but when these protections are impeded by diverse factors, and high protein is consumed, the disease can emerge.

Clostridium perfringens alpha toxin is a toxin produced by the bacterium Clostridium perfringens and is responsible for gas gangrene and myonecrosis in infected tissues. The toxin also possesses hemolytic activity.

Pore-forming proteins are usually produced by bacteria, and include a number of protein exotoxins but may also be produced by other organisms such as apple snails that produce perivitellin-2 or earthworms, who produce lysenin. They are frequently cytotoxic, as they create unregulated pores in the membrane of targeted cells.
A leukocidin is a type of cytotoxin created by some types of bacteria (Staphylococcus). It is a type of pore-forming toxin. The model for pore formation is step-wise. First, the cytotoxin’s “S” subunit recognizes specific protein-containing receptors, or an integrin on the host cell’s surface. The S subunit then recruits a second, “F” subunit, and the two subunits dimerize on the surface of the host’s cell. After dimerization, oligomerization occurs. Finally, the oligomers, consisting of alternating S and F subunits, undergo a significant structural change and form a beta-barrel, that pierces through the host cell’s lipid bilayer.

Alpha-toxin, also known as alpha-hemolysin (Hla), is the major cytotoxic agent released by bacterium Staphylococcus aureus and the first identified member of the pore forming beta-barrel toxin family. This toxin consists mostly of beta-sheets (68%) with only about 10% alpha-helices. The hly gene on the S. aureus chromosome encodes the 293 residue protein monomer, which forms heptameric units on the cellular membrane to form a complete beta-barrel pore. This structure allows the toxin to perform its major function, development of pores in the cellular membrane, eventually causing cell death.

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. Phospholipase C's role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.

Clostridium septicum is a gram positive, spore forming, obligate anaerobic bacterium.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxin. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.
Clostridium novyi (oedematiens) a Gram-positive, endospore- forming, obligate anaerobic bacteria of the class Clostridia. It is ubiquitous, being found in the soil and faeces. It is pathogenic, causing a wide variety of diseases in man and animals.
Hathewaya histolytica is a species of bacteria found in feces and the soil. It is a motile, gram-positive, aerotolerant anaerobe. H. histolytica is pathogenic in many species, including guinea pigs, mice, and rabbits, and humans. H. histolytica has been shown to cause gas gangrene, often in association with other bacteria species.
The Clostridial Cytotoxin (CCT) Family is a member of the RTX-toxin superfamily. There are currently 13 classified members belonging to the CCT family. A representative list of these proteins is available in the Transporter Classification Database. Homologues are found in a variety of Gram-positive and Gram-negative bacteria.










