
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.

Laser cooling includes several techniques where atoms, molecules, and small mechanical systems are cooled with laser light. The directed energy of lasers is often associated with heating materials, e.g. laser cutting, so it can be counterintuitive that laser cooling often results in sample temperatures approaching absolute zero. Laser cooling relies on the change in momentum when an object, such as an atom, absorbs and re-emits a photon. For example, if laser light illuminates a warm cloud of atoms from all directions and the laser's frequency is tuned below an atomic resonance, the atoms will be cooled. This common type of laser cooling relies on the Doppler effect where individual atoms will preferentially absorb laser light from the direction opposite to the atom's motion. The absorbed light is re-emitted by the atom in a random direction. After repeated emission and absorption of light the net effect on the cloud of atoms is that they will expand more slowly. The slower expansion reflects a decrease in the velocity distribution of the atoms, which corresponds to a lower temperature and therefore the atoms have been cooled. For an ensemble of particles, their thermodynamic temperature is proportional to the variance in their velocity. More homogeneous velocities between particles corresponds to a lower temperature. Laser cooling techniques combine atomic spectroscopy with the aforementioned mechanical effect of light to compress the velocity distribution of an ensemble of particles, thereby cooling the particles.
In physics and physical chemistry, time-resolved spectroscopy is the study of dynamic processes in materials or chemical compounds by means of spectroscopic techniques. Most often, processes are studied after the illumination of a material occurs, but in principle, the technique can be applied to any process that leads to a change in properties of a material. With the help of pulsed lasers, it is possible to study processes that occur on time scales as short as 10−16 seconds. All time-resolved spectra are suitable to be analyzed using the two-dimensional correlation method for a correlation map between the peaks.
In electromagnetism, Brillouin scattering, named after Léon Brillouin, refers to the interaction of light with the material waves in a medium. It is mediated by the refractive index dependence on the material properties of the medium; as described in optics, the index of refraction of a transparent material changes under deformation.
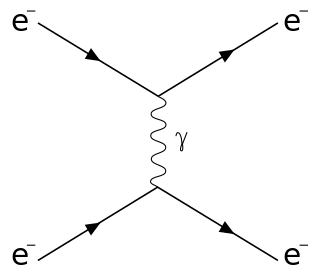
In physics, Raman scattering or the Raman effect is the inelastic scattering of photons by matter, meaning that there is both an exchange of energy and a change in the light's direction. Typically this effect involves vibrational energy being gained by a molecule as incident photons from a visible laser are shifted to lower energy. This is called normal Stokes-Raman scattering.

An optical parametric amplifier, abbreviated OPA, is a laser light source that emits light of variable wavelengths by an optical parametric amplification process. It is essentially the same as an optical parametric oscillator, but without the optical cavity.

An optical parametric oscillator (OPO) is a parametric oscillator that oscillates at optical frequencies. It converts an input laser wave with frequency into two output waves of lower frequency by means of second-order nonlinear optical interaction. The sum of the output waves' frequencies is equal to the input wave frequency: . For historical reasons, the two output waves are called "signal" and "idler", where the output wave with higher frequency is the "signal". A special case is the degenerate OPO, when the output frequency is one-half the pump frequency, , which can result in half-harmonic generation when signal and idler have the same polarization.
Ultrafast laser spectroscopy is a category of spectroscopic techniques using ultrashort pulse lasers for the study of dynamics on extremely short time scales. Different methods are used to examine the dynamics of charge carriers, atoms, and molecules. Many different procedures have been developed spanning different time scales and photon energy ranges; some common methods are listed below.

Resonance-enhanced multiphoton ionization (REMPI) is a technique applied to the spectroscopy of atoms and small molecules. In practice, a tunable laser can be used to access an excited intermediate state. The selection rules associated with a two-photon or other multiphoton photoabsorption are different from the selection rules for a single photon transition. The REMPI technique typically involves a resonant single or multiple photon absorption to an electronically excited intermediate state followed by another photon which ionizes the atom or molecule. The light intensity to achieve a typical multiphoton transition is generally significantly larger than the light intensity to achieve a single photon photoabsorption. Because of this, subsequent photoabsorption is often very likely. An ion and a free electron will result if the photons have imparted enough energy to exceed the ionization threshold energy of the system. In many cases, REMPI provides spectroscopic information that can be unavailable to single photon spectroscopic methods, for example rotational structure in molecules is easily seen with this technique.
Sum frequency generation spectroscopy (SFG) is a nonlinear laser spectroscopy technique used to analyze surfaces and interfaces. It can be expressed as a sum of a series of Lorentz oscillators. In a typical SFG setup, two laser beams mix at an interface and generate an output beam with a frequency equal to the sum of the two input frequencies, traveling in a direction allegedly given by the sum of the incident beams' wavevectors. The technique was developed in 1987 by Yuen-Ron Shen and his students as an extension of second harmonic generation spectroscopy and rapidly applied to deduce the composition, orientation distributions, and structural information of molecules at gas–solid, gas–liquid and liquid–solid interfaces. Soon after its invention, Philippe Guyot-Sionnest extended the technique to obtain the first measurements of electronic and vibrational dynamics at surfaces. SFG has advantages in its ability to be monolayer surface sensitive, ability to be performed in situ, and its capability to provide ultrafast time resolution. SFG gives information complementary to infrared and Raman spectroscopy.
The technique of vibrational analysis with scanning probe microscopy allows probing vibrational properties of materials at the submicrometer scale, and even of individual molecules. This is accomplished by integrating scanning probe microscopy (SPM) and vibrational spectroscopy. This combination allows for much higher spatial resolution than can be achieved with conventional Raman/FTIR instrumentation. The technique is also nondestructive, requires non-extensive sample preparation, and provides more contrast such as intensity contrast, polarization contrast and wavelength contrast, as well as providing specific chemical information and topography images simultaneously.
Rotating-polarization coherent anti-Stokes Raman spectroscopy, (RP-CARS) is a particular implementation of the coherent anti-Stokes Raman spectroscopy (CARS). RP-CARS takes advantage of polarization-dependent selection rules in order to gain information about molecule orientation anisotropy and direction within the optical point spread function.
Brillouin spectroscopy is an empirical spectroscopy technique which allows the determination of elastic moduli of materials. The technique uses inelastic scattering of light when it encounters acoustic phonons in a crystal, a process known as Brillouin scattering, to determine phonon energies and therefore interatomic potentials of a material. The scattering occurs when an electromagnetic wave interacts with a density wave, photon-phonon scattering.

AFM-IR or infrared nanospectroscopy is one of a family of techniques that are derived from a combination of two parent instrumental techniques. AFM-IR combines the chemical analysis power of infrared spectroscopy and the high-spatial resolution of scanning probe microscopy (SPM). The term was first used to denote a method that combined a tuneable free electron laser with an atomic force microscope equipped with a sharp probe that measured the local absorption of infrared light by a sample with nanoscale spatial resolution.

Cho Minhaeng is a South Korean scientist in researching physical chemistry, spectroscopy, and microscopy. He was director of the National Creative Research Initiative Center for Coherent Multidimensional Spectroscopy and is founding director of the Center for Molecular Spectroscopy and Dynamics in the Institute for Basic Science (IBS), located in Korea University.

Cavity optomechanics is a branch of physics which focuses on the interaction between light and mechanical objects on low-energy scales. It is a cross field of optics, quantum optics, solid-state physics and materials science. The motivation for research on cavity optomechanics comes from fundamental effects of quantum theory and gravity, as well as technological applications.
Stimulated Raman spectroscopy, also referred to as stimulated Raman scattering (SRS) is a form of spectroscopy employed in physics, chemistry, biology, and other fields. The basic mechanism resembles that of spontaneous Raman spectroscopy: a pump photon, of the angular frequency , which is scattered by a molecule has some small probability of inducing some vibrational transition, as opposed to inducing a simple Rayleigh transition. This makes the molecule emit a photon at a shifted frequency. However, SRS, as opposed to spontaneous Raman spectroscopy, is a third-order non-linear phenomenon involving a second photon—the Stokes photon of angular frequency —which stimulates a specific transition. When the difference in frequency between both photons resembles that of a specific vibrational transition the occurrence of this transition is resonantly enhanced. In SRS, the signal is equivalent to changes in the intensity of the pump and Stokes beams. The signals are typically rather low, of the order of a part in 10^5, thus calling for modulation-transfer techniques: one beam is modulated in amplitude and the signal is detected on the other beam via a lock-in amplifier. Employing a pump laser beam of a constant frequency and a Stokes laser beam of a scanned frequency allows for the unraveling of the spectral fingerprint of the molecule. This spectral fingerprint differs from those obtained by other spectroscopy methods such as Rayleigh scattering as the Raman transitions confer to different exclusion rules than those that apply for Rayleigh transitions.
Coherent Raman scattering (CRS) microscopy is a multi-photon microscopy technique based on Raman-active vibrational modes of molecules. The two major techniques in CRS microscopy are stimulated Raman scattering (SRS) and coherent anti-Stokes Raman scattering (CARS). SRS and CARS were theoretically predicted and experimentally realized in the 1960s. In 1982 the first CARS microscope was demonstrated. In 1999, CARS microscopy using a collinear geometry and high numerical aperture objective were developed in Xiaoliang Sunney Xie's lab at Harvard University. This advancement made the technique more compatible with modern laser scanning microscopes. Since then, CRS's popularity in biomedical research started to grow. CRS is mainly used to image lipid, protein, and other bio-molecules in live or fixed cells or tissues without labeling or staining. CRS can also be used to image samples labeled with Raman tags, which can avoid interference from other molecules and normally allows for stronger CRS signals than would normally be obtained for common biomolecules. CRS also finds application in other fields, such as material science and environmental science.












