Related Research Articles

In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons from which one hydrogen atom has been removed are functional groups called hydrocarbyls. Hydrocarbons are generally colorless and hydrophobic with only weak odors. Because of their diverse molecular structures, it is difficult to generalize further.

Infrared spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance on the vertical axis vs. frequency or wavelength on the horizontal axis. Typical units of frequency used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers, symbol μm, which are related to wave numbers in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. Two-dimensional IR is also possible as discussed below.

Spectroscopy is the study of the interaction between matter and electromagnetic radiation as a function of the wavelength or frequency of the radiation. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. We can also consider matter waves and acoustic waves as forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO).

Ultraviolet–visible spectroscopy or ultraviolet–visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible spectral regions. This means it uses light in the visible and adjacent ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, atoms and molecules undergo electronic transitions. Absorption spectroscopy is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.

Pyrolysis is the thermal decomposition of materials at elevated temperatures in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements pyro "fire" and lysis "separating".

Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.

Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths.

Thymol (also known as 2-isopropyl-5-methylphenol, IPMP) is a natural monoterpenoid phenol derivative of cymene, C10H14O, isomeric with carvacrol, found in oil of thyme, and extracted from Thymus vulgaris (common thyme), Ajwain and various other kinds of plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties. Thymol also provides the distinctive, strong flavor of the culinary herb thyme, also produced from T. vulgaris.
Devarda's alloy, is an alloy of aluminium (44% – 46%), copper (49% – 51%) and zinc (4% – 6%).

A flame test is an analytical procedure used in chemistry to detect the presence of certain elements, primarily metal ions, based on each element's characteristic emission spectrum. The color of flames in general also depends on temperature; see flame color.
The iodine value in chemistry is the mass of iodine in grams that is consumed by 100 grams of a chemical substance. Iodine numbers are often used to determine the amount of unsaturation in fats, oils and waxes. In fatty acids, unsaturation occurs mainly as doubles bonds which are very reactive towards halogens, the iodine in this case. Thus, the higher the iodine value, the more unsaturations are present in the fat. It can be seen from the table that coconut oil is very saturated, which means it is good for making soap. On the other hand, linseed oil is highly unsaturated, which makes it a drying oil, well suited for making oil paints.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. Similarly, biochemists use NMR to identify proteins and other complex molecules. Besides identification, NMR spectroscopy provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules. The most common types of NMR are proton and carbon-13 NMR spectroscopy, but it is applicable to any kind of sample that contains nuclei possessing spin.

Asphaltenes are molecular substances that are found in crude oil, along with resins, aromatic hydrocarbons, and saturates. The word "asphaltene" was coined by Boussingault in 1837 when he noticed that the distillation residue of some bitumens had asphalt-like properties. Asphaltenes in the form of asphalt or bitumen products from oil refineries are used as paving materials on roads, shingles for roofs, and waterproof coatings on building foundations.
Nuclear magnetic resonance spectroscopy of proteins is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and their complexes. The field was pioneered by Richard R. Ernst and Kurt Wüthrich at the ETH, and by Ad Bax, Marius Clore, and Angela Gronenborn at the NIH, among others. Structure determination by NMR spectroscopy usually consists of several phases, each using a separate set of highly specialized techniques. The sample is prepared, measurements are made, interpretive approaches are applied, and a structure is calculated and validated.
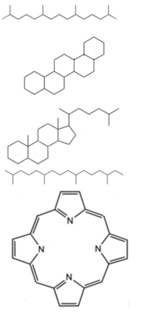
In chemistry and geology, biomarkers are any suite of complex organic compounds composed of carbon, hydrogen and other elements or heteroatoms such as oxygen, nitrogen and sulfur, which are found in crude oils, bitumen, petroleum source rock and eventually show simplification in molecular structure from the parent organic molecules found in all living organisms. Essentially, they are complex carbon-based molecules derived from formerly living organisms. Each biomarker is quite distinctive when compared to its counterparts, as the time required for organic matter to convert to crude oil is discreet. Most biomarkers also usually have high molecular mass.
Biogasoline/Biopetrol is gasoline produced from biomass such as algae. Like traditionally produced gasoline, it contains between 6 (hexane) and 12 (dodecane) carbon atoms per molecule and can be used in internal-combustion engines. Biogasoline is chemically different from biobutanol and bioethanol, as these are alcohols, not hydrocarbons.

Panicudine (6-hydroxy-11-deoxy-13-dehydrohetisane) is a C20-diterpene alkaloid of the hetisine type, first isolated from Aconitum paniculatum. It has empirical formula C20H25NO3 and a melting point of 249-250 °C. The structure was determined to be a hetisine type diterpene by noting infrared spectrum absorption bands of 3405 cm−1 (OH), 1718 (C=O), and 1650 (C=C), a proton magnetic resonance spectrum with "secondary hydroxy (4.02 ppm, m, 1H, W1/2 = 10 Hz), exomethylene (4.87 and 4.76 ppm, br.s, 1H each), and tertiary methyl (1.29 ppm, s, 3H) groups and the absence of N-methyl, N-ethyl, and methoxy groups." Additional ultraviolet spectrum and carbon-13 NMR data, confirmed by high resolution mass spectrometry, completed the determination of the structure.
Cadmium tetrafluoroborate is an ionic, chemical compound with the formula Cd(BF4)2. It is an ionic, crystalline solid, which is colorless and odorless. Cadmium tetrafluoroborate is most frequently used in the industrial production of high-strength steels, its purpose being to prevent hydrogen absorption, a source of post-production cracking of the metal, in the treated steels. Another application of the chemistry of cadmium tetrafluoroborate is fine tuning of the size of cadmium telluride nanomaterials.

Cooking oil is plant, animal, or synthetic fat used in frying, baking, and other types of cooking. It is also used in food preparation and flavouring not involving heat, such as salad dressings and bread dippings like bread dips, and may be called edible oil.

2,2-Dimethylpentane is one of the isomers of heptane. It is also called neoheptane as it contains the (CH3)3C grouping. It has the most extreme properties of the isomers.
References
- ↑ Dolomatov, M. Yu; Yarmukhametova, G. U. (May 2008). "Correlation of color characteristics with Conradson carbon residue and molecular weight of complex hydrocarbon media". Journal of Applied Spectroscopy. 75 (3): 433–438. Bibcode:2008JApSp..75..433D. doi:10.1007/s10812-008-9064-z. S2CID 97292617.
- ↑ Dolomatov, M. Yu.; Yarmukhametova, G. U. (July 2009). "Determining the mean molecular mass for crude oil and oil residues from color characteristics". Chemistry and Technology of Fuels and Oils. 45 (4): 288–293. doi:10.1007/s10553-009-0139-1. S2CID 95399426.
- ↑ Kalashchenko, N. V. (March 2006). "Normal and pathological color characteristics of human blood components". Journal of Applied Spectroscopy. 73 (2): 245–250. Bibcode:2006JApSp..73..245K. doi:10.1007/s10812-006-0065-5. S2CID 95426229.
- ↑ "Fenomen of paramagnetic shift of color characteristics in multicomponent hydrocarbon systems". International Journal of Theoretical and Applied Physics. June 2013.
- ↑ Dolomatov, M. Yu.; Domatov, L. V. (April 1988). "Rapid determination of carbon residue of heavy products from thermal breakdown". Chemistry and Technology of Fuels and Oils. 24 (4): 180–181. doi:10.1007/BF00725196. S2CID 93408560.
- ↑ Dolomatov, M. Yu.; Khashper, L. M.; Kuz'Mina, Z. F. (July 1991). "Spectroscopic method for determination of the average molecular weight". Chemistry and Technology of Fuels and Oils. 27 (7): 401–403. doi:10.1007/BF00725388. S2CID 97765609.
- ↑ Dolomatov, M. Yu.; Kuz'Mina, Z. F.; Lomakin, S. P.; Khashper, L. M. (September 1991). "Rapid determination of relative density of petroleum fractions". Chemistry and Technology of Fuels and Oils. 27 (9): 518–519. doi:10.1007/BF00718802. S2CID 95456324..
- ↑ Dolomatov, M. Yu.; Amirova, S. I.; Kuz'Mina, Z. F.; Lomakin, S. P. (October 1991). "Determination of the coking capacity of mixtures of high-molecular-weight organic compounds". Chemistry and Technology of Fuels and Oils. 27 (10): 580–582. doi:10.1007/BF00724546. S2CID 98008885.
- ↑ Dolomatov, M. Yu. (January 1995). "Application of electronic phenomenological spectroscopy in the identification and investigation of complex organic systems". Chemistry and Technology of Fuels and Oils. 31: 42–47. doi:10.1007/BF00727664. S2CID 98275956.
- ↑ Mukaeva, G. R. (May–June 1998). "Spectroscopic control of the properties of organic substances and materials by the property-absorption coefficient correlations". Journal of Applied Spectroscopy. 65 (3): 456–458. Bibcode:1998JApSp..65..456M. doi:10.1007/BF02675469. S2CID 95612479.
- ↑ Dolomatov, M. Yu.; Shulyakovskaya, D. O. (April 2013). "Determination of Physicochemical Properties of Multicomponent Hydrocarbon Systems Based on Integral Characteristics of Electronic Absorption Spectra". Chemistry and Technology of Fuels and Oils. 49 (2): 175–179. doi:10.1007/s10553-013-0428-6. S2CID 96717169.
- ↑ Dolomatov, M. Yu.; Shulyakovskaya, D. O.; Yarmukhametova, G. U.; Mukaeva, G. R. (June 2013). "Evaluation of physico-chemical properties of hydrocarbon systems based on spectrum-property and color-property correlations". Chemistry and Technology of Fuels and Oils. 49 (3): 273–280. doi:10.1007/s10553-013-0441-9. S2CID 94826739.
- ↑ Dolomatov, Mikhail Yurievich; Shulyakovskaya, Darya Olegovna; Mukaeva, Guzel Ragipovna; Paymurzina, Natalya Khalitovna (August 2012). "Testing amorphous, multi-component, organic dielectrics according to their electronic spectrums and color characteristics". Applied Physics Research. 4 (3). doi: 10.5539/apr.v4n3p83 .
- ↑ "Simple definition methods of electron structures of materials and molecules for nanoelectronics". Nanotech Europe 2009. September 2009.