
In chemistry, iron(III) refers to the element iron in its +3 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe3+.

Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being or copper(II) oxide or cupric oxide (CuO). This red-coloured solid is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles. Copper(I) oxide is found as the reddish mineral cuprite.

Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics.
Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ([CuCl4]2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate [Cu(CH3)2]−). The term cuprates derives from the Latin word for copper, cuprum. The term is mainly used in three contexts: oxide materials, anionic coordination complexes, and anionic organocopper compounds.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.
The Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.

Copper monosulfide is a chemical compound of copper and sulfur. It was initially thought to occur in nature as the dark indigo blue mineral covellite. However, it was later shown to be rather a cuprous compound, formula Cu+3S(S2). CuS is a moderate conductor of electricity. A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts. It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis and photovoltaics.
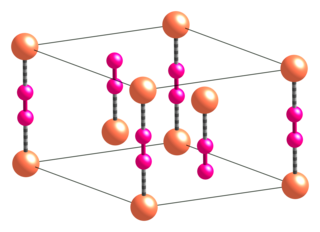
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.

Mercury(II) thiocyanate (Hg(SCN)2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding "snake" when ignited, an effect known as the Pharaoh's serpent.

Sodium thiocyanate (sometimes called sodium sulphocyanide) is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, it is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals. Thiocyanate salts are typically prepared by the reaction of cyanide with elemental sulfur:

Copper(I) fluoride or cuprous fluoride is an inorganic compound with the chemical formula CuF. Its existence is uncertain. It was reported in 1933 to have a sphalerite-type crystal structure. Modern textbooks state that CuF is not known, since fluorine is so electronegative that it will always oxidise copper to its +2 oxidation state. Complexes of CuF such as [(Ph3P)3CuF] are, however, known and well characterised.

Cobalt(II) thiocyanate is an inorganic compound with the formula Co(SCN)2. It is a layered coordination complex and its trihydrate Co(SCN)2(H2O)3 is used in the cobalt thiocyanate test (or Scott test) for detecting cocaine. The test has been responsible for widespread false positives and false convictions.

Lead(II) thiocyanate is a compound, more precisely a salt, with the formula Pb(SCN)2. It is a white crystalline solid, but will turn yellow upon exposure to light. It is slightly soluble in water and can be converted to a basic salt (Pb(CNS)2·Pb(OH)2 when boiled. Salt crystals may form upon cooling. Lead thiocyanate can cause lead poisoning if ingested and can adversely react with many substances. It has use in small explosives, matches, and dyeing.

Tetrakis(acetonitrile)copper(I) hexafluorophosphate is a salt with the formula [Cu(CH3CN)4]PF6. It is a colourless solid that is used in the synthesis of other copper complexes. The cation [Cu(CH3CN)4]+ is a well-known example of a transition metal nitrile complex.
Copper(I) nitrate is a proposed inorganic compound with formula of CuNO3. It has not been characterized by X-ray crystallography. It is the focus of one publication, which describes unsuccessful efforts to isolate the compound. Another nonexistent simple copper(I) compound derived from an oxyanion is cuprous perchlorate. On the other hand, cuprous sulfate is known.

Copper(II) thiocyanate (or cupric thiocyanate) is a coordination polymer with formula Cu(SCN)2. It is a black solid which slowly decomposes in moist air. It was first reported in 1838 by Carl Ernst Claus and its structure was determined first in 2018.

Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes.














