
Wilkinson's catalyst (chloridotris(triphenylphosphene)rhodium(I)) is a coordination complex of rhodium with the formula [RhCl(PPh3)3], where 'Ph' denotes a phenyl group. It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use.
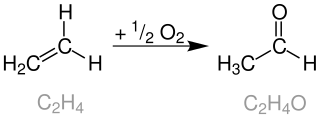
The Wacker process or the Hoechst-Wacker process refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride and copper(II) chloride as the catalyst. This chemical reaction was one of the first homogeneous catalysis with organopalladium chemistry applied on an industrial scale.
The Corey–House synthesis (also called the Corey–Posner–Whitesides–House reaction and other permutations) is an organic reaction that involves the reaction of a lithium diorganylcuprate () with an organic halide or pseudohalide () to form a new alkane, as well as an ill-defined organocopper species and lithium (pseudo)halide as byproducts.
The Ullmann reaction or Ullmann coupling, named after Fritz Ullmann, couples two aryl or alkyl groups with the help of copper. The reaction was first reported by Ullmann and his student Bielecki in 1901. It has been later shown that palladium and nickel can also be effectively used.

Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently by Masamune and Dauben and Whalen. A patent application published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio.
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.
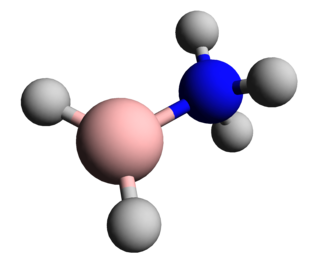
In chemistry, a boranylium ion is an inorganic cation with the chemical formula BR+
2, where R represents a non-specific substituent. Being electron-deficient, boranylium ions form adducts with Lewis bases. Boranylium ions have historical names that depend on the number of coordinated ligands:
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.
Diborane(2), also known as diborene, is an inorganic compound with the formula B2H2. The number 2 in diborane(2) indicates the number of hydrogen atoms bonded to the boron complex. There are other forms of diborane with different numbers of hydrogen atoms, including diborane(4) and diborane(6).

The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols.
In organic chemistry, the Fujiwara–Moritani reaction is a type of cross coupling reaction where an aromatic C-H bond is directly coupled to an olefinic C-H bond, generating a new C-C bond. This reaction is performed in the presence of a transition metal, typically palladium. The reaction was discovered by Yuzo Fujiwara and Ichiro Moritani in 1967. An external oxidant is required to this reaction to be run catalytically. Thus, this reaction can be classified as a C-H activation reaction, an oxidative Heck reaction, and a C-H olefination. Surprisingly, the Fujiwara–Moritani reaction was discovered before the Heck reaction.
The Mukaiyama hydration is an organic reaction involving formal addition of an equivalent of water across an olefin by the action of catalytic bis(acetylacetonato)cobalt(II) complex, phenylsilane and atmospheric oxygen to produce an alcohol with Markovnikov selectivity.
In organic chemistry, the Murai reaction is an organic reaction that uses C-H activation to create a new C-C bond between a terminal or strained internal alkene and an aromatic compound using a ruthenium catalyst. The reaction, named after Shinji Murai, was first reported in 1993. While not the first example of C-H activation, the Murai reaction is notable for its high efficiency and scope. Previous examples of such hydroarylations required more forcing conditions and narrow scope.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.
The Stahl oxidation is a copper-catalyzed aerobic oxidation of primary and secondary alcohols to aldehydes and ketones. Known for its high selectivity and mild reaction conditions, the Stahl oxidation offers several advantages over classical alcohol oxidations.
The inorganic imides are compounds containing an ion composed of nitrogen bonded to hydrogen with formula HN2−. Organic imides have the NH group, and two single or one double covalent bond to other atoms. The imides are related to the inorganic amides (H2N−), the nitrides (N3−) and the nitridohydrides (N3−•H−).
Metal-ligand cooperativity (MLC) is a mode of reactivity in which a metal and ligand of a complex are both involved in the bond breaking or bond formation of a substrate during the course of a reaction. This ligand is an actor ligand rather than a spectator, and the reaction is generally only deemed to contain MLC if the actor ligand is doing more than leaving to provide an open coordination site. MLC is also referred to as "metal-ligand bifunctional catalysis." Note that MLC is not to be confused with cooperative binding.

The nitro-Mannich reaction is the nucleophilic addition of a nitroalkane to an imine, resulting in the formation of a beta-nitroamine. With the reaction involving the addition of an acidic carbon nucleophile to a carbon-heteroatom double bond, the nitro-Mannich reaction is related to some of the most fundamental carbon-carbon bond forming reactions in organic chemistry, including the aldol reaction, Henry reaction and Mannich reaction.
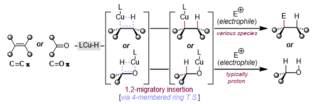
A hydrocupration is a chemical reaction whereby a ligated copper hydride species, reacts with a carbon-carbon or carbon-oxygen pi-system; this insertion is typically thought to occur via a four-membered ring transition state, producing a new copper-carbon or copper-oxygen sigma-bond and a stable (generally) carbon-hydrogen sigma-bond. In the latter instance (copper-oxygen), protonation (protodemetalation) is typical – the former (copper-carbon) has broad utility. The generated copper-carbon bond (organocuprate) has been employed in various nucleophilic additions to polar conjugated and non-conjugated systems and has also been used to forge new carbon-heteroatom bonds.

Tantalocene trihydride, or bis(η5-cyclopentadienyl)trihydridotantalum, is an organotanalum compound in the family of bent metallocenes consisting of two cyclopentadienyl rings and three hydrides coordinated to a tantalum center. Its formula is TaCp2H3, and it is a white crystalline compound that is sensitive to air. It is the first example of a molecular trihydride of a transition metal.




![Structure of [(Ph3P)CuH]6. Cu6H6P6.png](http://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Cu6H6P6.png/220px-Cu6H6P6.png)








