
Photosynthesis is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their activities. Photosynthetic organisms use intracellular organic compounds to store the chemical energy they produce in photosynthesis within organic compounds like sugars, glycogen, cellulose and starches. Photosynthesis is usually used to refer to oxygenic photosynthesis, a process that produces oxygen. To use this stored chemical energy, the organisms' cells metabolize the organic compounds through another process called cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth.

In botany, a stoma, also called a stomate, is a pore found in the epidermis of leaves, stems, and other organs, that controls the rate of gas exchange between the internal air spaces of the leaf and the atmosphere. The pore is bordered by a pair of specialized parenchyma cells known as guard cells that regulate the size of the stomatal opening.

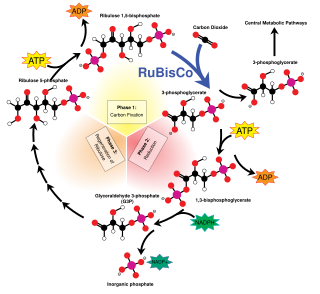
Ribulose-1,5-bisphosphate carboxylase/oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme involved in light-independent part of photosynthesis, including the carbon fixation by which atmospheric carbon dioxide is converted by plants and other photosynthetic organisms to energy-rich molecules such as glucose. It emerged approximately four billion years ago in primordial metabolism prior to the presence of oxygen on Earth. It is probably the most abundant enzyme on Earth. In chemical terms, it catalyzes the carboxylation of ribulose-1,5-bisphosphate.

C4 carbon fixation or the Hatch–Slack pathway is one of three known photosynthetic processes of carbon fixation in plants. It owes the names to the 1960s discovery by Marshall Davidson Hatch and Charles Roger Slack.

Photorespiration (also known as the oxidative photosynthetic carbon cycle or C2 cycle) refers to a process in plant metabolism where the enzyme RuBisCO oxygenates RuBP, wasting some of the energy produced by photosynthesis. The desired reaction is the addition of carbon dioxide to RuBP (carboxylation), a key step in the Calvin–Benson cycle, but approximately 25% of reactions by RuBisCO instead add oxygen to RuBP (oxygenation), creating a product that cannot be used within the Calvin–Benson cycle. This process lowers the efficiency of photosynthesis, potentially lowering photosynthetic output by 25% in C3 plants. Photorespiration involves a complex network of enzyme reactions that exchange metabolites between chloroplasts, leaf peroxisomes and mitochondria.

Ribulose 1,5-bisphosphate (RuBP) is an organic substance that is involved in photosynthesis, notably as the principal CO2 acceptor in plants. It is a colourless anion, a double phosphate ester of the ketopentose called ribulose. Salts of RuBP can be isolated, but its crucial biological function happens in solution. RuBP occurs not only in plants but in all domains of life, including Archaea, Bacteria, and Eukarya.
C3 carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, the other two being C4 and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phosphoglycerate through the following reaction:
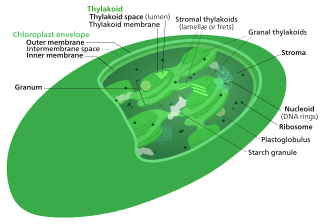
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation (redox) reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of CO2 to a sugar. There are three phases to the light-independent reactions, collectively called the Calvin cycle: carboxylation, reduction reactions, and ribulose 1,5-bisphosphate (RuBP) regeneration.
The photosynthetic efficiency is the fraction of light energy converted into chemical energy during photosynthesis in green plants and algae. Photosynthesis can be described by the simplified chemical reaction
Phosphoenolpyruvate carboxylase (also known as PEP carboxylase, PEPCase, or PEPC; EC 4.1.1.31, PDB ID: 3ZGE) is an enzyme in the family of carboxy-lyases found in plants and some bacteria that catalyzes the addition of bicarbonate (HCO3−) to phosphoenolpyruvate (PEP) to form the four-carbon compound oxaloacetate and inorganic phosphate:
Malate dehydrogenase (decarboxylating) (EC 1.1.1.39) or NAD-malic enzyme (NAD-ME) is an enzyme that catalyzes the chemical reaction

Malate dehydrogenase (oxaloacetate-decarboxylating) (NADP+) (EC 1.1.1.40) or NADP-malic enzyme (NADP-ME) is an enzyme that catalyzes the chemical reaction in the presence of a bivalent metal ion:

Pyruvate, phosphate dikinase, or PPDK is an enzyme in the family of transferases that catalyzes the chemical reaction

Portulacaria afra is a small-leaved succulent plant found in South Africa. These succulents commonly have a reddish stem and leaves that are green, but also a variegated cultivar is often seen in cultivation. They are simple to care for and make easy houseplants for a sunny location. In frost-free regions they may be used in outdoor landscaping.

Photosynthesis systems are electronic scientific instruments designed for non-destructive measurement of photosynthetic rates in the field. Photosynthesis systems are commonly used in agronomic and environmental research, as well as studies of the global carbon cycle.
The evolution of photosynthesis refers to the origin and subsequent evolution of photosynthesis, the process by which light energy is used to assemble sugars from carbon dioxide and a hydrogen and electron source such as water. It is believed that the pigments used for photosynthesis initially were used for protection from the harmful effects of light, particularly ultraviolet light. The process of photosynthesis was discovered by Jan Ingenhousz, a Dutch-born British physician and scientist, first publishing about it in 1779.

Photosynthesis converts carbon dioxide to carbohydrates via several metabolic pathways that provide energy to an organism and preferentially react with certain stable isotopes of carbon. The selective enrichment of one stable isotope over another creates distinct isotopic fractionations that can be measured and correlated among oxygenic phototrophs. The degree of carbon isotope fractionation is influenced by several factors, including the metabolism, anatomy, growth rate, and environmental conditions of the organism. Understanding these variations in carbon fractionation across species is useful for biogeochemical studies, including the reconstruction of paleoecology, plant evolution, and the characterization of food chains.

Alarm photosynthesis is a variation of photosynthesis where calcium oxalate crystals function as dynamic carbon pools, supplying carbon dioxide (CO2) to photosynthetic cells when stomata are partially or totally closed. This biochemical appendance of the photosynthetic machinery is a means to alleviate the perpetual plant dilemma of using atmospheric CO2 for photosynthesis and losing water vapor, or saving water and reducing photosynthesis. The function of alarm photosynthesis seems to be rather auxiliary to the overall photosynthetic performance. It supports a low photosynthetic rate, aiming at the maintenance and photoprotection of the photosynthetic apparatus rather than a substantial carbon gain.

Littorella uniflora (vernacular name: (American) shoreweed) is a species of aquatic flowering plant native to the Azores, Morocco, most of Europe excluding the dry southeast, Iceland, and the Faroes. It prefers to live mostly submerged in nutrient-poor freshwater habitats. When submerged, it draws CO2 mostly through its roots and uses a mix of crassulacean acid metabolism (CAM) and C3 carbon fixation for photosynthesis. If the water level drops and exposes the roots, it ceases using CAM.






















