Related Research Articles

Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.

Stathmin, also known as metablastin and oncoprotein 18 is a protein that in humans is encoded by the STMN1 gene.
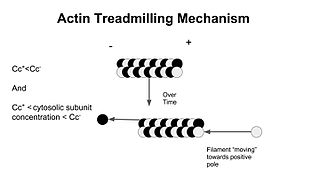
In molecular biology, treadmilling is a phenomenon observed within protein filaments of the cytoskeletons of many cells, especially in actin filaments and microtubules. It occurs when one end of a filament grows in length while the other end shrinks, resulting in a section of filament seemingly "moving" across a stratum or the cytosol. This is due to the constant removal of the protein subunits from these filaments at one end of the filament, while protein subunits are constantly added at the other end. Treadmilling was discovered by Wegner, who defined the thermodynamic and kinetic constraints. Wegner recognized that: “The equilibrium constant (K) for association of a monomer with a polymer is the same at both ends, since the addition of a monomer to each end leads to the same polymer.”; a simple reversible polymer can’t treadmill; ATP hydrolysis is required. GTP is hydrolyzed for microtubule treadmilling.
In enzymology, a tubulin—tyrosine ligase is an enzyme that catalyzes the chemical reaction
In enzymology, an alpha-tubulin N-acetyltransferase is an enzyme which is encoded by the ATAT1 gene.
Tubulin alpha-4A chain is a protein that in humans is encoded by the TUBA4A gene.
Tektins are cytoskeletal proteins found in cilia and flagella as structural components of outer doublet microtubules. They are also present in centrioles and basal bodies. They are polymeric in nature, and form filaments.
Tubulin alpha-1A chain is a protein that in humans is encoded by the TUBA1A gene.

Tubulin alpha-1B chain is a protein that in humans is encoded by the TUBA1B gene.
Tubulin alpha-3C/D chain is a protein that in humans is encoded by the TUBA3C gene.

Tubulin alpha-8 chain is a protein that in humans is encoded by the TUBA8 gene.
Tubulin alpha-1C chain is a protein that in humans is encoded by the TUBA1C gene.

TUBB1 is a gene that codes for the protein Tubulin beta-1 chain in humans.

Katanin p80 WD40-containing subunit B1 is a protein that in humans is encoded by the KATNB1 gene.

Gamma-tubulin complex component 2 is a protein that in humans is encoded by the TUBGCP2 gene. It is part of the gamma tubulin complex, which required for microtubule nucleation at the centrosome.

Katanin p60 ATPase-containing subunit A1 is an enzyme that in humans is encoded by the KATNA1 gene.

Pericentriolar material is a highly structured, dense mass of protein which makes up the part of the animal centrosome that surrounds the two centrioles. The PCM contains proteins responsible for microtubule nucleation and anchoring including γ-tubulin, pericentrin and ninein.
Protein acetylation are acetylation reactions that occur within living cells as drug metabolism, by enzymes in the liver and other organs. Pharmaceuticals frequently employ acetylation to enable such esters to cross the blood-brain barrier, where they are deacetylated by enzymes (carboxylesterases) in a manner similar to acetylcholine. Examples of acetylated pharmaceuticals are diacetylmorphine (heroin), acetylsalicylic acid (aspirin), THC-O-acetate, and diacerein. Conversely, drugs such as isoniazid are acetylated within the liver during drug metabolism. A drug that depends on such metabolic transformations in order to act is termed a prodrug.

Neurotubules are microtubules found in neurons in nervous tissues. Along with neurofilaments and microfilaments, they form the cytoskeleton of neurons. Neurotubules are undivided hollow cylinders that are made up of tubulin protein polymers and arrays parallel to the plasma membrane in neurons. Neurotubules have an outer diameter of about 23 nm and an inner diameter, also known as the central core, of about 12 nm. The wall of the neurotubules is about 5 nm in width. There is a non-opaque clear zone surrounding the neurotubule and it is about 40 nm in diameter. Like microtubules, neurotubules are greatly dynamic and the length of them can be adjusted by polymerization and depolymerization of tubulin.
References
Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation.
Aillaud C, Bosc C, Peris L, Bosson A, Heemeryck P, Van Dijk J, Le Friec J, Boulan B, Vossier F, Sanman LE, Syed S, Amara N, Couté Y, Lafanechère L, Denarier E, Delphin C, Pelletier L, Humbert S, Bogyo M, Andrieux A, Rogowski K, Moutin MJ.
Science. 2017 Dec 15;358(6369):1448-1453. doi: 10.1126/science.aao4165. Epub 2017 Nov 16.
PMID: 29146868
- ↑ Janke C, Bulinski JC (2011). "Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions". Nat Rev Mol Cell Biol. 12 (12): 773–86. doi:10.1038/nrm3227. PMID 22086369.
- ↑ Hallak ME, Rodriguez JA, Barra HS, Caputto R (1977). "Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin". FEBS Lett. 73 (2): 147–50. doi: 10.1016/0014-5793(77)80968-x . PMID 838053.
- ↑ Ersfeld K, Wehland J, Plessmann U, Dodemont H, Gerke V, Weber K (1993). "Characterization of the tubulin-tyrosine ligase". J Cell Biol. 120 (3): 725–32. doi:10.1083/jcb.120.3.725. PMC 2119537 . PMID 8093886.