The platypus, sometimes referred to as the duck-billed platypus, is a semiaquatic, egg-laying mammal endemic to eastern Australia, including Tasmania. The platypus is the sole living representative or monotypic taxon of its family (Ornithorhynchidae) and genus (Ornithorhynchus), though a number of related species appear in the fossil record.

Echidnas, sometimes known as spiny anteaters, are quill-covered monotremes belonging to the family Tachyglossidae. The four extant species of echidnas and the platypus are the only living mammals that lay eggs and the only surviving members of the order Monotremata. The diet of some species consists of ants and termites, but they are not closely related to the true anteaters of the Americas, which are xenarthrans. Echidnas live in Australia and New Guinea.

The Gymnotiformes are an order of teleost bony fishes commonly known as the Neotropical or South American knifefish. They have long bodies and swim using undulations of their elongated anal fin. Found almost exclusively in fresh water, these mostly nocturnal fish are capable of producing electric fields for navigation, communication, and, in the case of the electric eel, attack and defense. A few species are familiar to the aquarium trade, such as the black ghost knifefish, the glass knifefish, and the banded knifefish.

An electric fish is any fish that can generate electric fields. A fish that can generate electric fields is called electrogenic while a fish that has the ability to detect electric fields is called electroreceptive. Most electrogenic fish are also electroreceptive. The only group of electrogenic fish that are not electroreceptive are the stargazers, in the family Uranoscopidae. Electric fish species can be found both in the ocean and in freshwater rivers of South America (Gymnotiformes) and Africa (Mormyridae). These two groups of weak electric fish diverged and independently evolved similar ways to communicate and localise objects through producing and receiving electric fields. Many fish such as sharks, rays and catfishes can detect electric fields and are thus electroreceptive, but they are not classified as electric fish because they cannot generate electricity. Most common bony fish (teleosts), including most fish kept in aquaria or caught for food, are neither electrogenic nor electroreceptive. There are approximately 350 species of fish that have the ability to create an electric field that can be used for communication, navigation or predation. These electric fishes are found within the bony fishes (Osteichthyes) and the cartilaginous fishes (Chondrichthyes).

The black ghost knifefish is a tropical fish belonging to the ghost knifefish family (Apteronotidae). They originate in freshwater habitats in South America where they range from Venezuela to the Paraguay–Paraná River, including the Amazon Basin. They are popular in aquaria. The fish is all black except for two white rings on its tail, and a white blaze on its nose, which can occasionally extend into a stripe down its back. It moves mainly by undulating a long fin on its underside. It will grow to a maximum length of 50 cm (20 in).

Magnetoreception is a sense which allows an organism to detect the Earth's magnetic field. Animals with this sense include arthropods, molluscs, and vertebrates. The sense is mainly used for orientation and navigation, but it may help some animals to form regional maps. Experiments on migratory birds suggest that they make use of a cryptochrome protein in the eye, relying on the quantum radical pair mechanism to perceive magnetic fields. This effect is extremely sensitive to weak magnetic fields, and readily disturbed by radio-frequency interference, unlike a conventional iron compass.

The ampullae of Lorenzini are special sensing organs called electroreceptors, where they can form a network of mucus-filled pores. They are mostly found in cartilaginous fish ; however, they are also found in basal actinopterygians such as reedfish and sturgeon. Lungfish have also been reported to have them.

The Mormyridae, sometimes called "elephantfish", are a family of freshwater fish in the order Osteoglossiformes native to Africa. It is by far the largest family in the order with around 200 species. Members of the family can be popular, if challenging, aquarium species. These fish are also known for having large brain size and unusually high intelligence.

Peters's elephant-nose fish is an African freshwater elephantfish in the genus Gnathonemus. Other names in English include elephantnose fish, long-nosed elephant fish, and Ubangi mormyrid, after the Ubangi River. The Latin name petersii is probably for the German naturalist Wilhelm Peters. The fish uses electrolocation to find prey, and has the largest brain-to-body oxygen use ratio of all known vertebrates.

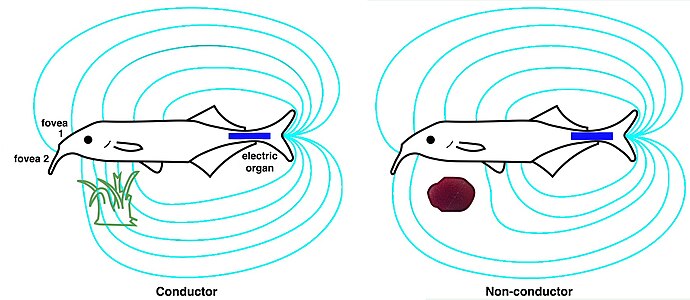
In biology, the electric organ is an organ common to all electric fish used for the purposes of creating an electric field. The electric organ is derived from modified nerve or muscle tissue. The electric discharge from this organ is used for navigation, communication, mating, defense and also sometimes for the incapacitation of prey.

A Knollenorgan is an electroreceptor in the skin of weakly electric fish of the family Mormyridae (Elephantfish) from Africa. The structure was first described by Viktor Franz (1921), a German anatomist unaware of its function. They are named after "Knolle", German for "tuberous root" which describes their structure.
Magnetic shark repellents utilize permanent magnets, which exploit the sensitivity of the Ampullae of Lorenzini in sharks and rays (electrosense). This organ is not found on bony fish (teleosts), therefore, this type of shark repellent is selective to sharks and rays. Permanent magnets do not require power input, making them practical for use in fisheries and as bycatch reduction devices. Sharkbanz, released in 2014, is a wearable commercially available device intended for recreational users. Its manufacturers cite numerous scientific papers which support the effectiveness of permanent magnets in a range of contexts. A field study of a range of shark deterrents in 2018 found that Sharkbanz were ineffective when used in a temperate oceanic setting with berley-attracted Great white sharks.
Sternarchogiton nattereri is a species of weakly electric knifefish in the family Apteronotidae. It is native to the Amazon River system and feeds on sponges. Unlike other members of the genus Sternarchogiton, there is pronounced sexual dimorphism in S. nattereri, with reproductively mature males developing strong external teeth on tips of their jaws. These males are so different from the females and juveniles that they were thought to be a different genus and species, the "tooth-lip knifefish" Oedemognathus exodon, for over 40 years.
Sternarchogiton porcinum is a species of weakly electric knifefish in the family Apteronotidae. It is native to deep river channels in the Río Huallaga, Río Napo, and Río Amazonas in Peru, and in the Río Orinoco in Venezuela. Many specimens once identified as S. porcinum from the Brazilian Amazon Basin and the Venezuelan Orinoco are now known to be a different species, S. preto.

Monotremes are prototherian mammals of the order Monotremata. They are one of the three main groups of living mammals, along with placentals (Eutheria) and marsupials (Metatheria). Monotremes are typified by structural differences in their brains, jaws, digestive tract, reproductive tract, and other body parts compared to the more common mammalian types. In addition, they lay eggs rather than bearing live young, but, like all mammals, the female monotremes nurse their young with milk.

The subfamily Mormyrinae contains all but one of the genera of the African freshwater fish family Mormyridae in the order Osteoglossiformes. They are often called elephantfish due to a long protrusion below their mouths used to detect buried invertebrates that is suggestive of a tusk or trunk. They can also be called tapirfish.

Jamming avoidance response (JAR) is a behavior performed by some species of weakly electric fish. The JAR occurs when two electric fish with wave discharges meet – if their discharge frequencies are very similar, each fish will shift its discharge frequency to increase the difference between the two fish's discharge frequencies. By doing this, both fish prevent jamming of their sense of electroreception.

Bioelectrogenesis is the generation of electricity by living organisms, a phenomenon that belongs to the science of electrophysiology. In biological cells, electrochemically active transmembrane ion channel and transporter proteins, such as the sodium-potassium pump, make electricity generation possible by maintaining a voltage imbalance from an electrical potential difference between the intracellular and extracellular space. The sodium-potassium pump simultaneously releases three sodium ions away from, and influxes two potassium ions towards, the intracellular space. This generates an electrical potential gradient from the uneven charge separation created. The process consumes metabolic energy in the form of ATP.

Hydrodynamic reception refers to the ability of some animals to sense water movements generated by biotic or abiotic sources. This form of mechanoreception is useful for orientation, hunting, predator avoidance, and schooling. Frequent encounters with conditions of low visibility can prevent vision from being a reliable information source for navigation and sensing objects or organisms in the environment. Sensing water movements is one resolution to this problem.
Most fish possess highly developed sense organs. Nearly all daylight fish have color vision that is at least as good as a human's. Many fish also have chemoreceptors that are responsible for extraordinary senses of taste and smell. Although they have ears, many fish may not hear very well. Most fish have sensitive receptors that form the lateral line system, which detects gentle currents and vibrations, and senses the motion of nearby fish and prey. Sharks can sense frequencies in the range of 25 to 50 Hz through their lateral line.