Denitrobacterium is a genus of Actinomycetota with a single species, in the family Coriobacteriaceae. Originally isolated from the bovine rumen, Denitrobacterium are non-motile and non-spore forming. The only described species in this genus is Denitrobacterium detoxificans. The specific niche of this bacterium in the bovine rumen is theorized to be the detoxification/metabolism of nitrotoxins and miserotoxin.

Lamium album, commonly called white nettle or white dead-nettle, is a flowering plant in the family Lamiaceae. It is native throughout Europe and Asia, growing in a variety of habitats from open grassland to woodland, generally on moist, fertile soils.

Malvin is a naturally occurring chemical of the anthocyanin family.

Aniba rosaeodora, also known as pau-rosa, is a species of Magnoliid tree in the family Lauraceae. Although sometimes wrongly referred to as rosewood this name is totally misleading; it is no tree of the genus Dalbergia. It grows in parts of the tropical rainforest of South America. It is an endangered species that sees exploitation for its essential oil.
Ocotea catharinensis is a member of the plant family Lauraceae. It is a slow-growing evergreen, a valuable hardwood tree of broad ecological importance, and it is threatened by habitat loss and by overexploitation for its timber and essential oils.

Karwinskia humboldtiana, commonly known as coyotillo, cacachila or Humboldt coyotillo, is a species of flowering shrub or small tree in the family Rhamnaceae. It is native to southern and western Texas in the United States as well as much of Mexico. The seeds and leaves of this plant contain the quinones eleutherin and 7-methoxyeleutherin and chrysophanol and β-amyrin in the fruits that are toxic to humans and livestock. The toxins typically induce paralysis, which is often followed by death. However, it often takes days or even weeks after consumption for the symptoms to manifest.

Myricitrin is a plant compound, the 3-O-α-L-rhamnopyranoside of myricetin.
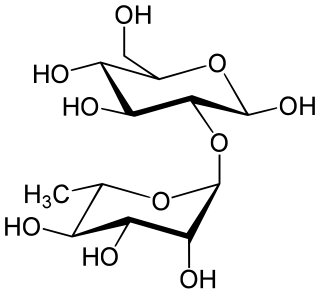
Neohesperidose is the disaccharide which is present in some flavonoids. It can be found in species of Typha.
Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

Brickellin is an O-methylated flavonol. It can be found in Brickellia veronicifolia.

Mearnsetin is an O-methylated flavonol. It can be found in Eucalyptus globulus and in Elaeocarpus lanceofolius. The compound has antioxidative properties.

Tellimagrandin II is the first of the ellagitannins formed from 1,2,3,4,6-pentagalloyl-glucose. It can be found in Geum japonicum and Syzygium aromaticum (clove).

A xanthonoid is a chemical natural phenolic compound formed from the xanthone backbone. Many members of the Clusiaceae contain xanthonoids.
Psoralea plicata is a herb species in the genus Psoralea found in Pakistan.
Erythrina orientalis is a plant species in the genus Erythrina. This plant is a climbing herb that grows up to 6 m long, and has compound leaves with petioles that are 5–6 cm long. Its leaflets emerge in groups of three, and are 7–9 cm long and 5–8 cm wide. The plant's young leaves, flowers and pods are consumed as vegetables.

Astringin is a stilbenoid, the 3-β-D-glucoside of piceatannol. It can be found in the bark of Picea sitchensis or Picea abies.

Violdelphin is an anthocyanin, a plant pigment, has been found in the purplish blue flower of Aconitum chinense, in the blue flowers in the genus Campanula and in the blue flowers of Delphinium hybridum. It is a flavenoid natural product, incorporating two p-hydroxy benzoic acid residues, one rutinoside and two glucosides associated with a delphinidin.
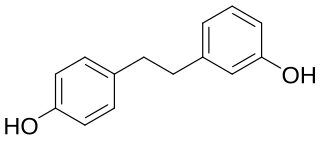
Lunularin is a dihydrostilbenoid found in common celery. It has also been found in the roots of Hydrangea macrophylla.

Hypolaetin is a flavone. It is the aglycone of hypolaetin 8-glucuronide, a compound found in the liverwort Marchantia berteroana. Hypolaetin 8-glucoside can be found in Sideritis leucantha.

Isorhapontin is a stilbenoid. It is the glucoside of isorhapontigenin. It can be found in mycorrhizal and non-mycorrhizal roots of Norway spruces, in the bark of Picea sitchensis or in white spruce.















