The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables. The name derives from the Latin word for "wood". Lignans are precursors to phytoestrogens. They may play a role as antifeedants in the defense of seeds and plants against herbivores.

Gmelina arborea,, locally known as gamhar, is a fast-growing deciduous tree in the family Lamiaceae.

Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a natural product of the coumarin family.
Neoflavonoids are a class of polyphenolic compounds. While flavonoids have the 2-phenylchromen-4-one backbone, neoflavonoids have the 4-phenylchromen backbone with no hydroxyl group substitution at position 2.

Pandava Vanavasam is a 1965 Indian Telugu-language Hindu mythological film directed by Kamalakara Kameswara Rao and written by Samudrala Sr. It stars N. T. Rama Rao and Savitri, with music composed by Ghantasala. It was produced by A. S. R. Anjaneyulu under the Madhavi Productions banner. The film was a major box office success, running for 175 days in theatres.

Gmelina is a genus of plants in the family Lamiaceae. It consists of about 35 species in Australia, New Guinea, New Caledonia, Southeast Asia, India and a few in Africa. Some species such as G. arborea have been planted and/or become naturalised in India, Africa and Australia. It was named by Carl Linnaeus in honour of botanist Johann Georg Gmelin.

Uppaluri Siva Ramachandra Murty, or U. S. R. Murty, is a Professor Emeritus of the Department of Combinatorics and Optimization, University of Waterloo.

Gunasundari Katha is a 1949 Indian Telugu language fantasy film produced and directed by K. V. Reddy, starring Sriranjani, Kasturi Siva Rao, Santha Kumari. The script was written by Pingali Nagendra Rao, K. V. Reddy, and Kamalakara Kameswara Rao. Kameswara Rao was also the associate director. William Shakespeare's play King Lear was the inspiration for the core plot. However the writers changed the tone from the tragedy of King Lear to a more entertaining one for the film. The film was commercially successful.

Dalbergichromene is a neoflavene, a type of neoflavonoid. Dalbergichromene can be extracted from the stem-bark and heartwood of Dalbergia sissoo. It has also been synthesized from vanillin.

Padandi Munduku: The Dandi March is a 1962 Telugu-language political thriller film directed by V. Madhusudhana Rao, and produced by Jaggayya. The film is touted to be the first Indian film based on the Salt March and India's independence movement as its core theme.

Enterolactone is a organic compound classified as an enterolignan. It is formed by the action of intestinal bacteria on plant lignan precursors present in the diet.

Raavu Balasaraswathi Devi is an Indian singer and actress who performed from 1930 to the 1960s in Telugu and Tamil cinema. She was the first light music singer on All India Radio and the first playback singer in the Telugu cinema.

Arboreol is an epoxylignan. Arboreol can be transformed by acid catalysis into gmelanone.

Lavendamycin is a naturally occurring chemical compound discovered in fermentation broth of the soil bacterium Streptomyces lavendulae. Lavendamycin has antibiotic properties and anti-proliferative effects against several cancer cell lines. The use of lavendamycin as a cytotoxic agent in cancer therapy failed due to poor water solubility and non-specific cytotoxicity. The study of lavendamycin-based analogs designed to overcome these liabilities has been an area of research.
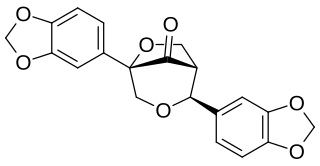
Gmelanone is a lignan found in the heartwood of Gmelina arborea. Arboreol can be transformed by acid catalysis into gmelanone.

Gmelinol is a lignan. (+)-Gmelinol can be isolated from the heartwood of Gmelina arborea. This compound, along with four other chemicals also found in the same species, (+)-7′-O-ethyl arboreol, (+)-paulownin, (+)-epieudesmin and (−)-β-sitosterol, shows antifungal activity against Trametes versicolor.

Kurukshetram is a 1977 Indian Telugu-language Hindu mythological film produced by A. S. R. Anjaneyulu and directed by Kamalakara Kameswara Rao. It stars Krishna, Sobhan Babu, Krishnam Raju and Kaikala Satyanarayana while Jamuna, Vijaya Nirmala, Anjali Devi and Gummadi played other important roles.
Ganugapati Sree Rama Subba Rao is an Indian natural product chemist and a former chair of the department of sciences at the Indian Institute of Science (IISc). He is known for his researches on dihydroaromatics obtained through Birch reduction of aromatic compounds and is an elected fellow of the Indian National Science Academy, and the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1982, for his contributions to chemical sciences.
Barry Ramachandra Rao was an Indian space physicist and the vice chairman of the University Grants Commission of India. Known for his pioneering research in radio physics, Rao was a Member of Parliament of the Rajya Sabha and an elected fellow of all the three major Indian science academies viz. Indian Academy of Sciences, Indian National Science Academy and National Academy of Sciences, India. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Physical Sciences in 1965.

DY Centauri is a variable star in the constellation Centaurus. From its brightness, it is estimated to be 7000 parsecs (23000 light-years) away from Earth.
















