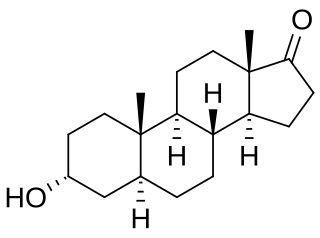
Androsterone, or 3α-hydroxy-5α-androstan-17-one, is an endogenous steroid hormone, neurosteroid, and putative pheromone. It is a weak androgen with a potency that is approximately 1/7 that of testosterone. Androsterone is a metabolite of testosterone and dihydrotestosterone (DHT). In addition, it can be converted back into DHT via 3α-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase, bypassing conventional intermediates such as androstanedione and testosterone, and as such, can be considered to be a metabolic intermediate in its own right.
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family, and was invented in 1988. It is most closely related in structure to the GABA antagonist flumazenil, although its effects are somewhat different. It is classified as a high-potency benzodiazepine due to its high affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile. In particular bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome. Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5 and α6 subunit containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5GABAA benzodiazepine receptor complexes.

Androstenol, also known as 5α-androst-16-en-3α-ol, is a 16-androstene class steroidal pheromone and neurosteroid in humans and other mammals, notably pigs. It possesses a characteristic musk-like odor.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

Androstadienone, or androsta-4,16-dien-3-one, is a 16-androstene class endogenous steroid that has been described as having potent pheromone-like activities in humans. The compound is synthesized from androstadienol by 3β-hydroxysteroid dehydrogenase, and can be converted into androstenone by 5α-reductase, which can subsequently be converted into 3α-androstenol or 3β-androstenol by 3-ketosteroid reductase.

Etifoxine, sold under the trade name Stresam among others, is a nonbenzodiazepine anxiolytic agent, primarily indicated for short-term management of adjustment disorder, specifically instances of situational depression accompanied by anxiety, such as stress-induced anxiety. Administration is by mouth. Side effects associated with etifoxine use include slight drowsiness, headache, skin eruptions, and allergic reactions. In rare cases, etifoxine has been linked to severe skin and liver toxicity, as well as menstrual bleeding between periods. Unlike benzodiazepines, etifoxine does not cause sedation or lack of coordination. Etifoxine acts as a GABAA receptor positive allosteric modulator and as a ligand for translocator proteins. Both mechanisms are conjectured to contribute to its anxiolytic properties.

Ocinaplon is an anxiolytic drug in the pyrazolopyrimidine family of drugs. Other pyrazolopyrimidine drugs include zaleplon and indiplon.

Abecarnil (ZK-112,119) is an anxiolytic drug from the β-Carboline family. It is one of a relatively recently developed class of medicines known as the nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It is a partial agonist acting selectively at the benzodiazepine site of the GABAA receptor.

Androstadienol, or androsta-5,16-dien-3β-ol, is a 16-androstene class endogenous steroid, pheromone, and chemical intermediate to several other pheromones that is found in the sweat of both men and women.
Pherines, also known as vomeropherines, are odorless synthetic neuroactive steroids that engage nasal chemosensory receptors and induce dose-dependent and reversible pharmacological and behavioral effects. Pherines target human chemosensory receptors and possibly other receptors such as the GABAA receptor and influence central nervous system activity.

Itruvone, also known as pregn-4-en-20-yn-3-one, is a vomeropherine which is under development by VistaGen Therapeutics as a nasal spray for the treatment of major depressive disorder.

Cetadiol, also known as androst-5-ene-3β,16α-diol, is a drug described as a "steroid tranquilizer" which was briefly investigated as a treatment for alcoholism in the 1950s. It is an androstane steroid and analogue of 5-androstenediol (androst-5-ene-3β,17β-diol) and 16α-hydroxy-DHEA (androst-5-ene-3β,16α-diol-17-one), but showed no androgenic or myotrophic activity in animal bioassays. The drug was reported in 1956 and studied until 1958.
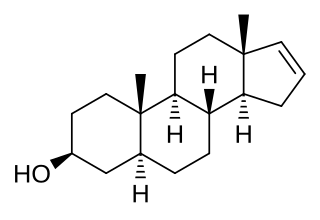
3β-Androstenol, also known as 5α-androst-16-en-3β-ol, is a naturally occurring mammalian pheromone known to be present in humans and pigs. It is thought to play a role in axillary odor. It is produced from androstenone via the enzyme 3β-hydroxysteroid dehydrogenase. Unlike its C3α epimer 3α-androstenol, 3β-androstenol shows no potentiation of the GABAA receptor or anticonvulsant activity.

Zuranolone, sold under the brand name Zurzuvae, is a medication used for the treatment of postpartum depression. It is taken by mouth.

Darigabat is a GABAergic medication which is under development for the treatment of photosensitive epilepsy, focal onset seizures, panic disorder, and other anxiety disorders. It was also under development for the treatment of generalized anxiety disorder and chronic lower back pain, but development for these indications was discontinued. It is taken via oral administration.
Deuterated etifoxine is a deuterated drug which is under development for the treatment of anxiety disorders and mood disorders. It was originated by GABA Therapeutics and is under development by GABA Therapeutics and ATAI Life Sciences. Deuterated etifoxine is a deuterated form of etifoxine (Stresam) with improved pharmacokinetic properties, for instance a longer elimination half-life and duration of action. Etifoxine has been widely used as an anxiolytic for many decades. Etifoxine and deuterated etifoxine are GABAA receptor positive allosteric modulators (GABAkines) and ligands of the translocator protein (TSPO), both of which may contribute to anxiolytic effects. The TSPO promotes steroidogenesis of inhibitory neurosteroids such as allopregnanolone, which act as potent GABAA receptor positive allosteric modulators, and hence interactions with the TSPO can also indirectly potentiate the GABAA receptor. The precise isotopic substitution of deuterated etifoxine has not yet been disclosed. As of January 2023, deuterated etifoxine is in phase 1 clinical trials for anxiety disorders and preclinical development for mood disorders.















