Related Research Articles

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Noradrenergic and specific serotonergic antidepressants (NaSSAs) are a class of psychiatric drugs used primarily as antidepressants. They act by antagonizing the α2-adrenergic receptor and certain serotonin receptors such as 5-HT2A and 5-HT2C, but also 5-HT3, 5-HT6, and/or 5-HT7 in some cases. By blocking α2-adrenergic autoreceptors and heteroreceptors, NaSSAs enhance adrenergic and serotonergic neurotransmission in the brain involved in mood regulation, notably 5-HT1A-mediated transmission. In addition, due to their blockade of certain serotonin receptors, serotonergic neurotransmission is not facilitated in unwanted areas, which prevents the incidence of many side effects often associated with selective serotonin reuptake inhibitor (SSRI) antidepressants; hence, in part, the "specific serotonergic" label of NaSSAs.
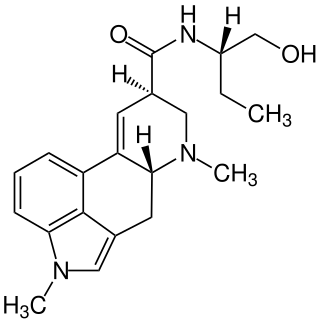
Methysergide, sold under the brand names Deseril and Sansert, is a monoaminergic medication of the ergoline and lysergamide groups which is used in the prophylaxis and treatment of migraine and cluster headaches. It has been withdrawn from the market in the United States and Canada due to adverse effects. It is taken by mouth.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.
The orexin receptor (also referred to as the hypocretin receptor) is a G-protein-coupled receptor that binds the neuropeptide orexin. There are two variants, OX1 and OX2, each encoded by a different gene (HCRTR1, HCRTR2).

Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.
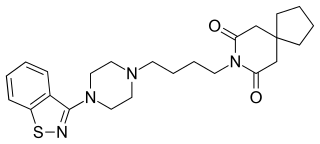
Tiospirone (BMY-13,859), also sometimes called tiaspirone or tiosperone, is an atypical antipsychotic of the azapirone class. It was investigated as a treatment for schizophrenia in the late 1980s and was found to have an effectiveness equivalent to those of typical antipsychotics in clinical trials but without causing extrapyramidal side effects. However, development was halted and it was not marketed. Perospirone, another azapirone derivative with antipsychotic properties, was synthesized and assayed several years after tiospirone. It was found to be both more potent and more selective in comparison and was commercialized instead.
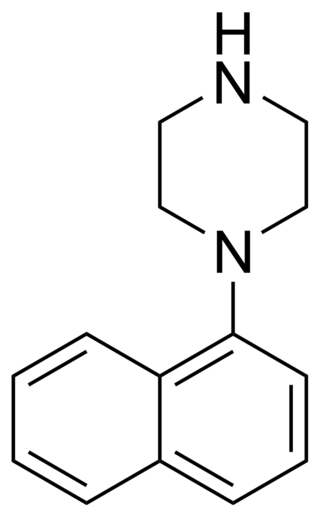
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors, while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors. It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors, and may bind to 5-HT4 and the SERT as well. In animals it produces effects including hyperphagia, hyperactivity, and anxiolysis, of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.
A serotonin modulator and stimulator (SMS), sometimes referred to more simply as a serotonin modulator, is a type of drug with a multimodal action specific to the serotonin neurotransmitter system. To be precise, SMSs simultaneously modulate one or more serotonin receptors and inhibit the reuptake of serotonin. The term was created to describe the mechanism of action of the serotonergic antidepressant vortioxetine, which acts as a serotonin reuptake inhibitor (SRI), agonist of the 5-HT1A receptor, and antagonist of the 5-HT3 and 5-HT7 receptors. However, it can also technically be applied to vilazodone, which is an antidepressant as well and acts as an SRI and 5-HT1A receptor partial agonist.

Amesergide is a serotonin receptor antagonist of the ergoline and lysergamide families related to methysergide which was under development by Eli Lilly and Company for the treatment of a variety of conditions including depression, anxiety, schizophrenia, male sexual dysfunction, migraine, and thrombosis but was never marketed. It reached phase II clinical trials for the treatment of depression, erectile dysfunction, and premature ejaculation prior to the discontinuation of its development.

JNJ-61393215 is an orexin antagonist medication which is under development for the treatment of depression and anxiety disorders. It is an orally active compound and acts as a selective antagonist of the orexin OX1 receptor (1-SORA). Preliminary clinical findings suggest that JNJ-61393215 may have anti-panic effects in humans. As of November 2021, JNJ-61393215 is in phase 2 clinical trials for the treatment of major depressive disorder and is in the preclinical stage of development for treatment of panic disorder, while no further development has been reported for treatment of other anxiety disorders. The drug was originated and developed by Janssen Pharmaceuticals.
Deuterated etifoxine is a deuterated drug which is under development for the treatment of anxiety disorders and mood disorders. It was originated by GABA Therapeutics and is under development by GABA Therapeutics and ATAI Life Sciences. Deuterated etifoxine is a deuterated form of etifoxine (Stresam) with improved pharmacokinetic properties, for instance a longer elimination half-life and duration of action. Etifoxine has been widely used as an anxiolytic for many decades. Etifoxine and deuterated etifoxine are GABAA receptor positive allosteric modulators (GABAkines) and ligands of the translocator protein (TSPO), both of which may contribute to anxiolytic effects. The TSPO promotes steroidogenesis of inhibitory neurosteroids such as allopregnanolone, which act as potent GABAA receptor positive allosteric modulators, and hence interactions with the TSPO can also indirectly potentiate the GABAA receptor. The precise isotopic substitution of deuterated etifoxine has not yet been disclosed. As of January 2023, deuterated etifoxine is in phase 1 clinical trials for anxiety disorders and preclinical development for mood disorders.
References
- 1 2 Garakani, Amir; Murrough, James W.; Iosifescu, Dan V. (2014). "Advances in Psychopharmacology for Anxiety Disorders". FOCUS. 12 (2): 152–162. doi: 10.1176/appi.focus.12.2.152 . ISSN 1541-4094.
- 1 2 Doggrell, S. A. (2007). Novel drugs and products in neuroscience. Drugs of the Future, 32(11), 1007-1017. https://journals.prous.com/journals/servlet/xmlxsl/pk_journals.xml_summary_pr?p_JournalId=2&p_RefId=3853&p_IsPs=Y
- ↑ Sessa B (2017). "MDMA and PTSD treatment: "PTSD: From novel pathophysiology to innovative therapeutics"". Neurosci. Lett. 649: 176–180. doi:10.1016/j.neulet.2016.07.004. PMID 27394687.
- ↑ Philipps, Dave (29 November 2016). "F.D.A. Agrees to New Trials for Ecstasy as Relief for PTSD Patients" . Retrieved 1 June 2017.
- ↑ "JNJ 42165279 - AdisInsight". adisinsight.springer.com. Retrieved 2019-03-02.
- ↑ "A Safety and Efficacy Study of JNJ-42165279 in Participants With Social Anxiety Disorder | Clinical Research Trial Listing ( Claustrophobia ) ( NCT02432703 )". www.centerwatch.com. Retrieved 2019-03-02.
- ↑ Rupprecht R, Pradhan AK, Kufner M, Brunner LM, Nothdurfter C, Wein S, Schwarzbach J, Puig X, Rupprecht C, Rammes G (December 2022). "Neurosteroids and translocator protein 18 kDa (TSPO) in depression: implications for synaptic plasticity, cognition, and treatment options". Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-022-01532-3. PMID 36574032.