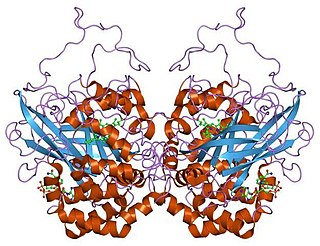
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.

Cytochrome c peroxidase, or CCP, is a water-soluble heme-containing enzyme of the peroxidase family that takes reducing equivalents from cytochrome c and reduces hydrogen peroxide to water:
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called comproportionation, also known as synproportionation.
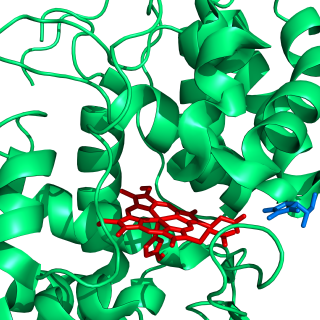
Ascorbate peroxidase (or L-ascorbate peroxidase, APX) (EC 1.11.1.11) is an enzyme that catalyzes the chemical reaction
In enzymology, a long-chain-alcohol dehydrogenase (EC 1.1.1.192) is an enzyme that catalyzes the chemical reaction
Long-chain alcohol oxidase is one of two enzyme classes that oxidize long-chain or fatty alcohols to aldehydes. It has been found in certain Candida yeast, where it participates in omega oxidation of fatty acids to produce acyl-CoA for energy or industrial use, as well as in other fungi, plants, and bacteria.
Chloride peroxidase (EC 1.11.1.10) is a family of enzymes that catalyzes the chlorination of organic compounds. This enzyme combines the inorganic substrates chloride and hydrogen peroxide to produce the equivalent of Cl+, which replaces a proton in hydrocarbon substrate:
In enzymology, a lignin peroxidase (EC 1.11.1.14) is an enzyme that catalyzes the chemical reaction
In enzymology, a manganese peroxidase (EC 1.11.1.13) is an enzyme that catalyzes the chemical reaction

In enzymology, a NADH peroxidase (EC 1.11.1.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a NADPH peroxidase (EC 1.11.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a phospholipid-hydroperoxide glutathione peroxidase (EC 1.11.1.12) is an enzyme that catalyzes the chemical reaction
Haem peroxidases (or heme peroxidases) are haem-containing enzymes that use hydrogen peroxide as the electron acceptor to catalyse a number of oxidative reactions. Most haem peroxidases follow the reaction scheme:

A selenenic acid is an organoselenium compound and an oxoacid with the general formula RSeOH, where R ≠ H. It is the first member of the family of organoselenium oxoacids, which also include seleninic acids and selenonic acids, which are RSeO2H and RSeO3H, respectively. Selenenic acids derived from selenoenzymes are thought to be responsible for the antioxidant activity of these enzymes. This functional group is sometimes called SeO-selenoperoxol.
Versatile peroxidase (EC 1.11.1.16, VP, hybrid peroxidase, polyvalent peroxidase) is an enzyme with systematic name reactive-black-5:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
Glutathione amide-dependent peroxidase (EC 1.11.1.17) is an enzyme with systematic name glutathione amide:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
Catalase-peroxidase (EC 1.11.1.21, katG (gene)) is an enzyme with systematic name donor:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
- donor + H2O2 ⇌ oxidized donor + 2 H2O
- 2 H2O2 ⇌ O2 + 2 H2O
Fatty-acid peroxygenase is an enzyme with systematic name fatty acid:hydroperoxide oxidoreductase (RH-hydroxylating). This enzyme catalyses the following chemical reaction

Eosinophil peroxidase is an enzyme found within the eosinophil granulocytes, innate immune cells of humans and mammals. This oxidoreductase protein is encoded by the gene EPX, expressed within these myeloid cells. EPO shares many similarities with its orthologous peroxidases, myeloperoxidase (MPO), lactoperoxidase (LPO), and thyroid peroxidase (TPO). The protein is concentrated in secretory granules within eosinophils. Eosinophil peroxidase is a heme peroxidase, its activities including the oxidation of halide ions to bacteriocidal reactive oxygen species, the cationic disruption of bacterial cell walls, and the post-translational modification of protein amino acid residues.







