
The development of fishes is unique in some specific aspects compared to the development of other animals.

The development of fishes is unique in some specific aspects compared to the development of other animals.
Most bony fish eggs are referred to as telolecithal which means that most of the egg cell cytoplasm is yolk. [1] The yolky end of the egg (the vegetal pole) remains homogenous while the other end (the animal pole) undergoes cell division. [2] Cleavage, or initial cell division, can only occur in a region called the blastodisc, a yolk free region located at the animal pole of the egg. The fish zygote is meroblastic, meaning the early cell divisions are not complete. This type of meroblastic cleavage is called discoidal because only the blastodisc becomes the embryo. [1] In fish, waves of calcium released direct the process of cell division by coordinating the mitotic apparatus with the actin cytoskeleton, propagating cell division along the surface, assists in deepening the cleavage furrow, and finally heals the membrane after separation of blastomeres. [3]
The fate of the first cells, called blastomeres, is determined by its location. This contrasts with the situation in some other animals, such as mammals, in which each blastomere can develop into any part of the organism. [2] Fish embryos go through a process called mid-blastula transition which is observed around the tenth cell division in some fish species. Once zygotic gene transcription starts, slow cell division begins and cell movements are observable. [4] During this time three cell populations become distinguished. The first population is the yolk syncytial layer. This layer forms when the cells at the vegetal pole of the blastoderm combine with the yolk cell underneath it. Later in development the yolk syncytial layer will be important in directing cell movements of gastrulation. The second cell population is the enveloping layer which is made of superficial cells from the blastoderm that eventually form a single epithelial cell layer. [1] This layer functions in protection by allowing the embryo to develop in a hypotonic solution so the cell will not burst. [5] Finally, the third set of blastomeres are the deep cells. These deep cells are located between the enveloping layer and the yolk syncytial layer and eventually give rise to the embryo proper. [1]
Once blastoderm cells have covered almost half of the yolk cell, thickening throughout the margin of deep cells occurs. The thickening is referred to as the germ ring and is made up of a superficial layer, the epiblast which will become ectoderm, and an inner layer called the hypoblast which will become endoderm and mesoderm. [6] As the blastoderm cells undergo epiboly around the yolk the internalization of cells at the blastoderm margin start to form hypoblast. Presumptive ectoderm or epiblast cells do not internalize but the deep cells (inner layer of cells) do and they become the mesoderm and endoderm. As the hypoblast cells move inward future mesoderm (hypoblast cells) start to move vegetally and proliferate but later in development these cells alter their direction and start moving towards the animal pole. However, endodermal precursors seem to lack a pattern and move randomly over the yolk. [7]
Once the egg has become multicellular and positioned its germ layers with ectoderm on the outside, mesoderm in the middle, and endoderm on the inside body axes have to be determined for proper development. [8] A dorsal-ventral axis has to form and major proteins involved are BMP and Wnts. Both proteins are made in the ventral and lateral portions of the developing embryo. BMP2B induces cells to have ventral and lateral fates while factors such as chordin can block BMPs to dorsalize the tissue. Wnt8 induces ventral, lateral, and posterior regions of embryonic tissue. Wnt also has inhibitors like noggin to allow for the formation of dorsal tissue. In order to aid in proper development fish have an organizer center called the Nieuwkoop center. [9] Anterior and posterior axis formation seems to be the result of interplay of FGFs, Wnt, and retinoic acid. FGFs, retinoic acid, and Wnts are required to turn on posterior genes. [8]
Neurulation, the formation of the central nervous system, is different in fishes than in most other chordates. Convergence and extension in the epiblast recruits presumptive neural cells from the epiblast towards the midline where they form a neural keel. A neural keel is a band of neural precursors that develops a slit like lumen to eventually become the neural tube. [1] The neural tube begins as a solid cord formed from the ectoderm. This cord then sinks into the embryo and becomes hollow, forming the neural tube. This process contrasts with the process in other chordates, which occurs by an infolding of the ectoderm to form a hollow tube. [10]
Throughout the years advances in research have shown that neural formation relies on interactions between extrinsic signaling factors and intrinsic transcription factors. Extrinsic signals involved are BMP, Wnt, and FGF and intrinsic transcription factors like SoxB1 related genes. Secreted proteins such as BMP and its antagonist Noggin and chordin act permissively to establish the fate of neural tissue in the dorsal ectoderm and enables the formation of the neural plate. [11]
Sex determination is variable in fish from environmental factors like temperature to genetic mechanisms. Some fish have XX/XY chromosomes and others have ZZ/ZW. So far one gene in specific, DMRT1bY, has been described as a sex determining gene. This gene is expressed before gonads develop and differentiate. Mutations in this gene lead to sex reversal from male to female. While this gene plays a major role in sex determination in some fish species other species have variations of this gene as well as some versions of the Sox gene as seen in zebrafish. [12] Many species of fishes are hermaphrodites. Some, such as the painted comber (Serranus scriba), are synchronous hermaphrodites. These fish have both ovaries and testes and can produce both eggs and sperm at the same time. Others are sequential hermaphrodites. These fishes start life as one sex and undergo a genetically programmed sex change at some point during development. Their gonads have both ovarian and testicular tissues, with one type of tissue predominant while the fish belongs to the corresponding gender. [13]

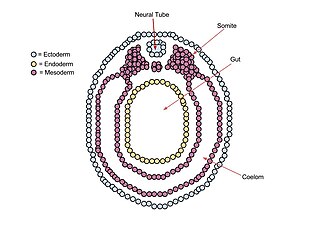
The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

The blastocoel, also spelled blastocoele and blastocele, and also called cleavage cavity, or segmentation cavity is a fluid-filled or yolk-filled cavity that forms in the blastula during very early embryonic development. At this stage in mammals the blastula develops into the blastocyst containing an inner cell mass, and outer trophectoderm.
A germ layer is a primary layer of cells that forms during embryonic development. The three germ layers in vertebrates are particularly pronounced; however, all eumetazoans produce two or three primary germ layers. Some animals, like cnidarians, produce two germ layers making them diploblastic. Other animals such as bilaterians produce a third layer between these two layers, making them triploblastic. Germ layers eventually give rise to all of an animal's tissues and organs through the process of organogenesis.
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation form the internal organs of the organism.

In developmental biology, animal embryonic development, also known as animal embryogenesis, is the developmental stage of an animal embryo. Embryonic development starts with the fertilization of an egg cell (ovum) by a sperm cell, (spermatozoon). Once fertilized, the ovum becomes a single diploid cell known as a zygote. The zygote undergoes mitotic divisions with no significant growth and cellular differentiation, leading to development of a multicellular embryo after passing through an organizational checkpoint during mid-embryogenesis. In mammals, the term refers chiefly to the early stages of prenatal development, whereas the terms fetus and fetal development describe later stages.
A neurula is a vertebrate embryo at the early stage of development in which neurulation occurs. The neurula stage is preceded by the gastrula stage; consequentially, neurulation is preceded by gastrulation. Neurulation marks the beginning of the process of organogenesis.
The primitive node is the organizer for gastrulation in most amniote embryos. In birds it is known as Hensen's node, and in amphibians it is known as the Spemann-Mangold organizer. It is induced by the Nieuwkoop center in amphibians, or by the posterior marginal zone in amniotes including birds.

The primitive streak is a structure that forms in the early embryo in amniotes. In amphibians, the equivalent structure is the blastopore. During early embryonic development, the embryonic disc becomes oval shaped, and then pear-shaped with the broad end towards the anterior, and the narrower region projected to the posterior. The primitive streak forms a longitudinal midline structure in the narrower posterior (caudal) region of the developing embryo on its dorsal side. At first formation, the primitive streak extends for half the length of the embryo. In the human embryo, this appears by stage 6, about 17 days.

In amniote embryonic development, the epiblast is one of two distinct cell layers arising from the inner cell mass in the mammalian blastocyst, or from the blastula in reptiles and birds, the other layer is the hypoblast. It drives the embryo proper through its differentiation into the three primary germ layers, ectoderm, mesoderm and endoderm, during gastrulation. The amniotic ectoderm and extraembryonic mesoderm also originate from the epiblast.

Mesenchyme is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone. The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo.
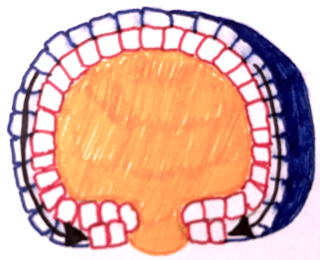
Epiboly describes one of the five major types of cell movements that occur in the gastrulation stage of embryonic development of some organisms. Epiboly is the spreading and thinning of the ectoderm while the endoderm and mesoderm layers move to the inside of the embryo.

The bilaminar embryonic disc, bilaminar blastoderm or embryonic disc is the distinct two-layered structure of cells formed in an embryo. In the development of the human embryo this takes place by day eight. It is formed when the inner cell mass, also known as the embryoblast, forms a bilaminar disc of two layers, an upper layer called the epiblast and a lower layer called the hypoblast, which will eventually form into fetus. These two layers of cells are stretched between two fluid-filled cavities at either end: the primitive yolk sac and the amniotic sac.
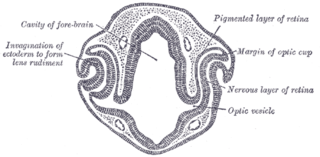
Eye formation in the human embryo begins at approximately three weeks into embryonic development and continues through the tenth week. Cells from both the mesodermal and the ectodermal tissues contribute to the formation of the eye. Specifically, the eye is derived from the neuroepithelium, surface ectoderm, and the extracellular mesenchyme which consists of both the neural crest and mesoderm.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks has 23 stages.

In amniote embryology, the hypoblast is one of two distinct layers arising from the inner cell mass in the mammalian blastocyst, or from the blastodisc in reptiles and birds. The hypoblast gives rise to the yolk sac, which in turn gives rise to the chorion.

In avian gastrulation, Koller's sickle is a local thickening of cells at the posterior edge of the upper layer of the area pellucida called the epiblast. Koller's sickle is crucial for avian development, due to its critical role in inducing the differentiation of various avian body parts. Koller's sickle induces primitive streak and Hensen's node, which are major components of avian gastrulation. Avian gastrulation is a process by which developing cells in an avian embryo move relative to one another in order to form the three germ layers.

In Xenopus laevis, the specification of the three germ layers occurs at the blastula stage. Great efforts have been made to determine the factors that specify the endoderm and mesoderm. On the other hand, only a few examples of genes that are required for ectoderm specification have been described in the last decade. The first molecule identified to be required for the specification of ectoderm was the ubiquitin ligase Ectodermin ; later, it was found that the deubiquitinating enzyme, FAM/USP9x, is able to overcome the effects of ubiquitination made by Ectodermin in Smad4. Two transcription factors have been proposed to control gene expression of ectodermal specific genes: POU91/Oct3/4 and FoxIe1/Xema. A new factor specific for the ectoderm, XFDL156, has shown to be essential for suppression of mesoderm differentiation from pluripotent cells.
This article is about the role of Fibroblast Growth Factor Signaling in Mesoderm Formation.