Related Research Articles
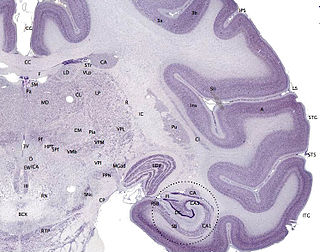
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is part of the brain responsible for cognition.

Reelin, encoded by the RELN gene, is a large secreted extracellular matrix glycoprotein that helps regulate processes of neuronal migration and positioning in the developing brain by controlling cell–cell interactions. Besides this important role in early development, reelin continues to work in the adult brain. It modulates synaptic plasticity by enhancing the induction and maintenance of long-term potentiation. It also stimulates dendrite and dendritic spine development and regulates the continuing migration of neuroblasts generated in adult neurogenesis sites like the subventricular and subgranular zones. It is found not only in the brain but also in the liver, thyroid gland, adrenal gland, Fallopian tube, breast and in comparatively lower levels across a range of anatomical regions.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

Colpocephaly is a cephalic disorder involving the disproportionate enlargement of the occipital horns of the lateral ventricles and is usually diagnosed early after birth due to seizures. It is a nonspecific finding and is associated with multiple neurological syndromes, including agenesis of the corpus callosum, Chiari malformation, lissencephaly, and microcephaly. Although the exact cause of colpocephaly is not known yet, it is commonly believed to occur as a result of neuronal migration disorders during early brain development, intrauterine disturbances, perinatal injuries, and other central nervous system disorders. Individuals with colpocephaly have various degrees of motor disabilities, visual defects, spasticity, and moderate to severe intellectual disability. No specific treatment for colpocephaly exists, but patients may undergo certain treatments to improve their motor function or intellectual disability.

Lissencephaly is a set of rare brain disorders whereby the whole or parts of the surface of the brain appear smooth. It is caused by defective neuronal migration during the 12th to 24th weeks of gestation resulting in a lack of development of brain folds (gyri) and grooves (sulci). It is a form of cephalic disorder. Terms such as agyria and pachygyria are used to describe the appearance of the surface of the brain.
In vertebrates, a neuroblast or primitive nerve cell is a postmitotic cell that does not divide further, and which will develop into a neuron after a migration phase. In invertebrates such as Drosophila, neuroblasts are neural progenitor cells which divide asymmetrically to produce a neuroblast, and a daughter cell of varying potency depending on the type of neuroblast. Vertebrate neuroblasts differentiate from radial glial cells and are committed to becoming neurons. Neural stem cells, which only divide symmetrically to produce more neural stem cells, transition gradually into radial glial cells. Radial glial cells, also called radial glial progenitor cells, divide asymmetrically to produce a neuroblast and another radial glial cell that will re-enter the cell cycle.

In neuroanatomy, a gyrus is a ridge on the cerebral cortex. It is generally surrounded by one or more sulci. Gyri and sulci create the folded appearance of the brain in humans and other mammals.

Stellate cells are neurons in the central nervous system, named for their star-like shape formed by dendritic processes radiating from the cell body. Many stellate cells are GABAergic and are located in the molecular layer of the cerebellum. Stellate cells are derived from dividing progenitor cells in the white matter of postnatal cerebellum. Dendritic trees can vary between neurons. There are two types of dendritic trees in the cerebral cortex, which include pyramidal cells, which are pyramid shaped and stellate cells which are star shaped. Dendrites can also aid neuron classification. Dendrites with spines are classified as spiny, those without spines are classified as aspinous. Stellate cells can be spiny or aspinous, while pyramidal cells are always spiny. Most common stellate cells are the inhibitory interneurons found within the upper half of the molecular layer in the cerebellum. Cerebellar stellate cells synapse onto the dendritic trees of Purkinje cells and send inhibitory signals. Stellate neurons are sometimes found in other locations in the central nervous system; cortical spiny stellate cells are found in layer IVC of the primary visual cortex. In the somatosensory barrel cortex of mice and rats, glutamatergic (excitatory) spiny stellate cells are organized in the barrels of layer 4. They receive excitatory synaptic fibres from the thalamus and process feed forward excitation to 2/3 layer of the primary visual cortex to pyramidal cells. Cortical spiny stellate cells have a 'regular' firing pattern. Stellate cells are chromophobes, that is cells that does not stain readily, and thus appears relatively pale under the microscope.
Pachygyria is a congenital malformation of the cerebral hemisphere. It results in unusually thick convolutions of the cerebral cortex. Typically, children have developmental delay and seizures, the onset and severity depending on the severity of the cortical malformation. Infantile spasms are common in affected children, as is intractable epilepsy.
Germinal matrix hemorrhage is a bleeding into the subependymal germinal matrix with or without subsequent rupture into the lateral ventricle. Such intraventricular hemorrhage can occur due to perinatal asphyxia in preterm neonates.

Intraventricular hemorrhage (IVH), also known as intraventricular bleeding, is a bleeding into the brain's ventricular system, where the cerebrospinal fluid is produced and circulates through towards the subarachnoid space. It can result from physical trauma or from hemorrhagic stroke.
The development of the nervous system in humans, or neural development or neurodevelopment involves the studies of embryology, developmental biology, and neuroscience to describe the cellular and molecular mechanisms by which the complex nervous system forms in humans, develops during prenatal development, and continues to develop postnatally.

Homeobox protein EMX1 is a protein that in humans is encoded by the EMX1 gene. The transcribed EMX1 gene is a member of the EMX family of transcription factors. The EMX1 gene, along with its family members, are expressed in the developing cerebrum. EMX1 plays a role in specification of positional identity, the proliferation of neural stem cells, differentiation of layer-specific neuronal phenotypes and commitment to a neuronal or glial cell fate.

The ganglionic eminence (GE) is a transitory structure in the development of the nervous system that guides cell and axon migration. It is present in the embryonic and fetal stages of neural development found between the thalamus and caudate nucleus.
Gyrification is the process of forming the characteristic folds of the cerebral cortex.

T-box, brain, 1 is a transcription factor protein important in vertebrate embryo development. It is encoded by the TBR1 gene. This gene is also known by several other names: T-Brain 1, TBR-1, TES-56, and MGC141978. TBR1 is a member of the TBR1 subfamily of T-box family transcription factors, which share a common DNA-binding domain. Other members of the TBR1 subfamily include EOMES and TBX21. TBR1 is involved in the differentiation and migration of neurons and is required for normal brain development. TBR1 interacts with various genes and proteins in order to regulate cortical development, specifically within layer VI of the developing six-layered human cortex. Studies show that TBR1 may play a role in major neurological diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and autism spectrum disorder (ASD).

The anatomy of the cerebellum can be viewed at three levels. At the level of gross anatomy, the cerebellum consists of a tightly folded and crumpled layer of cortex, with white matter underneath, several deep nuclei embedded in the white matter, and a fluid-filled ventricle in the middle. At the intermediate level, the cerebellum and its auxiliary structures can be broken down into several hundred or thousand independently functioning modules or compartments known as microzones. At the microscopic level, each module consists of the same small set of neuronal elements, laid out with a highly stereotyped geometry.
Corticogenesis is the process during which the cerebral cortex of the brain is formed as part of the development of the nervous system of mammals including its development in humans. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.
Cajal–Retzius cells are a heterogeneous population of morphologically and molecularly distinct reelin-producing cell types in the marginal zone/layer I of the developmental cerebral cortex and in the immature hippocampus of different species and at different times during embryogenesis and postnatal life.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). It occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.
References
- ↑ Nadarajah, Bagirathy; Parnavelas, John G. (1 June 2002). "Modes of neuronal migration in the developing cerebral cortex". Nature Reviews Neuroscience. 3 (6): 423–432. doi:10.1038/nrn845. ISSN 1471-0048. PMID 12042877. S2CID 38910547.
- ↑ Nadarajah, B. (2003-06-01). "Neuronal Migration in the Developing Cerebral Cortex: Observations Based on Real-time Imaging". Cerebral Cortex. 13 (6): 607–611. doi: 10.1093/cercor/13.6.607 . ISSN 1047-3211. PMID 12764035.
- ↑ Friocourt, Gaëlle; Kanatani, Shigeaki; Tabata, Hidenori; Yozu, Masato; Takahashi, Takao; Antypa, Mary; Raguénès, Odile; Chelly, Jamel; Férec, Claude (2008-05-28). "Cell-Autonomous Roles of ARX in Cell Proliferation and Neuronal Migration during Corticogenesis". Journal of Neuroscience. 28 (22): 5794–5805. doi:10.1523/JNEUROSCI.1067-08.2008. ISSN 0270-6474. PMC 6670801 . PMID 18509041.
- ↑ Brouwer, AJ; Groenendaal, F; Benders, MJ; de Vries, LS (2014). "Early and late complications of germinal matrix-intraventricular haemorrhage in the preterm infant: what is new?". Neonatology. 106 (4): 296–303. doi: 10.1159/000365127 . PMID 25171657. S2CID 3476273.